��Ŀ����
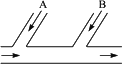
ij�����ų��ķ�ˮ�к�0.012 mol��L-1������̬�Ⱥ�0.002 mol��L-1��H+��Ϊ�˳�ȥ��ˮ������̬���ȣ�����ʹ��ˮ��Ϊ���ԣ������������ͼ��ʾ������ 
�ڷ�ˮ�ų��ܵ�A��B���ֱ�ע��һ�������ķ��ռ���Һ������������Һ��
�����
��1��A��B��Ӧ��������ʵĻ�ѧʽ��A_________��B_________��
��2��A��B������Ӧ�����ӷ���ʽ��
A��________________________________________________________________��
B��________________________________________________________________��
��3��ÿ����1 m3�ĸ÷�ˮ�������0.05 mol��L-1��Na2SO3��Һ������Ƕ��٣�
��4����ȥ�Ⱥ�ķ�ˮ��H+��Ũ��ԼΪ���٣�
��1��Na2SO3 NaOH ��2��Cl2+![]() +H2O
+H2O![]() 2H++
2H++![]() +2Cl- H++OH-
+2Cl- H++OH-![]() H2O
H2O
��3��240 L ��4��0.021 mol��L-1

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ