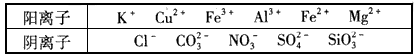��Ŀ����
��ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩���Ҹ����ӵ����ʵ�������ȶ�Ϊ0��0lmol��

������ԭ��Һ�м���KSCN��Һ�������Ա仯��
������ԭ��Һ�м������������,���������ɣ���Һ������������䡣
������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
�Իش���������
��1��������ԭ��Һ���ȼ�����������ᣬ�ټ���KSCN��Һ��������__________________���ù������漰�����ӷ���ʽ��________________��__________________��
��2����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù���Ϊ____________________��д��ѧʽ����
��3����ԭ��Һ�м����������������ˮ���ռ������������岢ʹ����ǡ�ó���������������������ˮ���У�����������ͨ��___________mLO2�����������ָ��״��������ʹ��Һ������������
��amolCu2S��bmol FeSͶ�뵽v L c mol��L��ϡ�����У���ַ�Ӧ������NO���壬���ó�����Һ���Կ���Cu��NO3��2��Fe��NO3��3��H2SO4�Ļ����Һ����Ӧ��δ����ԭ����������ʵ���Ϊ ___________mol����ͬʱ����a��b��v��c�ı���ʽ��ʾ����
����֪CH4��g����2O2��g�� CO2��g����2H2O��l�� ��H1��a kJ��mol�����㷴ӦCH4��g����4NO��g��
CO2��g����2H2O��l�� ��H1��a kJ��mol�����㷴ӦCH4��g����4NO��g�� 2N2��g����CO2��g����2H2O��l�����ʱ��H2������Ҫ����ij��Ӧ���ʱ��H3������Ӧ�и����ʻ�ѧ������֮��Ϊ���������ʱ��H3��b kJ��mol����÷�Ӧ���Ȼ�ѧ����ʽΪ______________________________________��
2N2��g����CO2��g����2H2O��l�����ʱ��H2������Ҫ����ij��Ӧ���ʱ��H3������Ӧ�и����ʻ�ѧ������֮��Ϊ���������ʱ��H3��b kJ��mol����÷�Ӧ���Ȼ�ѧ����ʽΪ______________________________________��
�ݴ˼������H2��_____________kJ��mol���ú�a��b��ʽ�ӱ�ʾ����
I����1�������ݲ�������Һ��ɣ�Ѫ����ɫ��2�֣���ֻҪ������Һ��ɣ�Ѫ����ɫ���÷֣�
3Fe2++4H++NO3-=3Fe3++NO��+2H2O��Fe3++3SCN-= Fe ��SCN��3(д�ɿ�����Ÿ���)��2�֣�
��2��Fe2O3��CuO��2�֣���©дһ�ֵ�һ�֣��д�д�����֣�(����������Ҳ��)
��3��56��2�֣�
��. 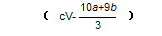 ��2�֣�
��2�֣�
��. N2(g) + O2(g)��2NO(g) ��H3 = b kJ/mol ��2�֣� a - 2b��2�֣�
��2NO(g)��N2(g) + O2(g) ��H3 = b kJ/mol a +2b
��������
�������������������Ƴ���Һ�к��е���������Ӧ��Fe2+��NO3-
��Cu2+ ��SO42-��Cl-�����Իش����⣨1������2��Ӧ����Fe��OH��2�ڼ���ʱ����Fe��OH��3��Cu��OH��2��Fe��OH��3������ʱ����Fe2O3��CuO����3����Ϊ4NO + 3O2 + 2H2O = 4HNO3,����Fe2+����Ϊ0.01mol���������O2������������õ��ӵ�ʧ�غ�����Ƴ�������������Ϊ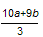 ���������������ȥ����������������������������ø�˹���ɣ������㷴ӦCH4��g����4NO��g��
���������������ȥ����������������������������ø�˹���ɣ������㷴ӦCH4��g����4NO��g��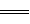 2N2��g����CO2��g����2H2O��l�������ǻ���Ҫ֪��N2��NO�Ĺ�ϵ������N2(g) + O2(g)��2NO(g) ��H3 = b kJ/mol����H2=��H1-2��H3
2N2��g����CO2��g����2H2O��l�������ǻ���Ҫ֪��N2��NO�Ĺ�ϵ������N2(g) + O2(g)��2NO(g) ��H3 = b kJ/mol����H2=��H1-2��H3
���㣺�������ӵĵ���غ�����ƶϣ����������ʼ䷴Ӧʱ�ĵ���ת�ƺͻ�ѧ����ʽ���Ȼ�ѧ����ʽ����д��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
|
������ |
SO42-��NO3-��Cl- |
|
������ |
Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
|
������ |
SO42-��NO3-��Cl- |
|
������ |
Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ��Իش���������
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��. �����������壨FeC2O4��2H2O����̼��﮺Ͷ�������������и��·�Ӧ���Ʊ�﮵�ص��������Ϲ�������ﮣ�Li2FeSiO4��������������������������н������ط������������ͼ��ʾ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�����,��ش��������⣺

��5����������������̼Ԫ�صĻ��ϼ�Ϊ��
��6��A��B������Ӧ�Ļ�ѧ����ʽΪ ��
��7����ȷ�о�������B��Cʵ���Ƿ��������еģ�ÿһ��ֻ�ͷ�һ�����壬�ڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����һ���ͷŵ����廯ѧʽΪ�� ���ͷŵڶ�������ʱ����Ӧ�Ļ�ѧ����ʽΪ ��
ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ��������________��
��2��ԭ��Һ�к��е���������________��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ________��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ________��