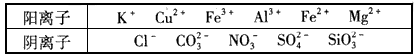��Ŀ����
ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ��������________��
��2��ԭ��Һ�к��е���������________��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ________��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ________��
�������������ԭ��Һ�м���KSCN��Һ�������Ա仯����˵����Һ��û�������ӡ�����ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䣬�����Һ��һ�����������ӡ��ܲ������ݵ�ֻ����NO3�������������±���ԭ����NO�����һ�������л�ԭ�������������ӣ�ͬʱ������NO3������������Һ�м���BaCl2��Һ���а�ɫ�������ɣ���˵��������SO42-���������ӵ����ʵ�����Ϊ1mol���������Һ�ĵ����Կ�֪����Һ��һ�������������ӡ�����ֻ�ܺ���5�����ӣ�����������ӵĵ������4mol��֪������һ����������ͭ���ӡ�
��1��������������NO3���ܰ�Fe2����������Fe3��������������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ����������Һ��ΪѪ��ɫ��
��2���������Ϸ�����֪��ԭ��Һ�к��е���������Fe2+��Cu2+��
��3���������ᣬ���������Ե�NO3-�ͻ�ԭ�Ե�Fe2+����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ3Fe2++4H++NO3-��3Fe3++NO��+2H2O��
��4��ԭ��Һ��������������Fe2+��Cu2+������ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�����CuO��Fe2O3��������������ӵ����ʵ�����Ϊ1mol��֪��m��CuO����1mol��80g/mol=80g��m��Fe2O3����0.5mol��160g/mol��80g�����ù��������Ϊ80g+80g��160.0g��
���㣺�������Ӽ��顢����������ԭ��Ӧ����д�Լ��йؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
|
������ |
SO42-��NO3-��Cl- |
|
������ |
Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
|
������ |
SO42-��NO3-��Cl- |
|
������ |
Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ��Իش���������
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��. �����������壨FeC2O4��2H2O����̼��﮺Ͷ�������������и��·�Ӧ���Ʊ�﮵�ص��������Ϲ�������ﮣ�Li2FeSiO4��������������������������н������ط������������ͼ��ʾ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�����,��ش��������⣺

��5����������������̼Ԫ�صĻ��ϼ�Ϊ��
��6��A��B������Ӧ�Ļ�ѧ����ʽΪ ��
��7����ȷ�о�������B��Cʵ���Ƿ��������еģ�ÿһ��ֻ�ͷ�һ�����壬�ڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����һ���ͷŵ����廯ѧʽΪ�� ���ͷŵڶ�������ʱ����Ӧ�Ļ�ѧ����ʽΪ ��
ij��Һ�н����±������е�5������(������ˮ�ĵ��뼰���ӵ�ˮ��)���Ҹ����ӵ����ʵ�����Ϊ0.1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ������������
������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䣻
������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
�Իش��������⣺
��1������ԭ��Һ���ȼ����������ᣬ�ٵ���KSCN��Һ��ʵ��������________��ʵ��������������Ӧ�����ӷ���ʽ��________��
��2��ԭ��Һ��������������(д���ӷ���)________.
��3������ԭ��Һ�м�������NaOH��Һ����ַ�Ӧ����һ��ʱ�䣬���ˡ�ϴ�ӡ����գ��������ù����������________g��