��Ŀ����
(8��)Ϊ����ʵ����ģ���ö�����������ˮ�������ᣬ�ֽ�������ʵ�飺
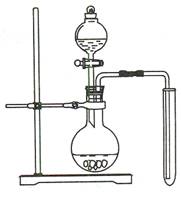 ʵ��I���Ʊ����ռ�һ�Թܶ�������
ʵ��I���Ʊ����ռ�һ�Թܶ�������
(1)ijͬѧ����ͼװ���Ʊ����ռ�һ�Թܶ���������װ�õ�ȱ����(ֻ��һ��ȱ�㼴��)
��
ʵ��II�������ܶ��ʹ�Թ��еĶ���������ˮ����
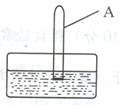
(2)��һ֧���������������Թ�A������ʢ��ˮ��ˮ����(��ͼ)��������ζ��Թܣ��۲쵽�������ǣ� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
(3)�ڸ�װ�õĻ����ϣ��������ʵ�飬Ҫ���ܶ��ʹ�Թ��еĶ���������ˮ���գ�ʵ�鲽���� ��
(I)û��β������װ�ã�������NO2�ݳ����¿�����Ⱦ�� (2��)
(������ʱ���Ʒ�Ӧ�Ŀ�ʼ��ֹͣ���˷�ҩƷ���ҹ�����NO2�ݳ����¿�����Ⱦ��)
(2)����ɫ��������ʧ��ˮλ���������ˮ���������Թܵ�2/3����ɫ��������Թܵ�1��3��![]() (2��)
(2��)
(���Թ���Һ��������ԼΪ�Թ������2��3�������ɺ���ɫ��Ϊ��ɫ��)
3NO2+H2O��2HNO3+NO w.w.w.k.s.5 u.c.o.m (2��)
[��ѧʽ�������֣�û����ƽ��1��]
(3)������������ͨ���Թ�A�С� (2��)

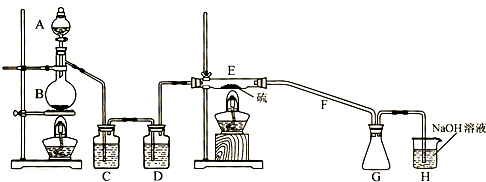
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
(A)��ʯ�� (B)����ʳ��ˮ (C)Ũ���� (D)����̼��������Һ
(2)�Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�____________________________��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ_______ɫ | ������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | _______________________________ |
| Ȼ����Һ����ɫ��Ϊ_______ɫ | _______________________________ |
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
| A����ʯ�� | B������ʳ��ˮ | C��Ũ���� | D������̼��������Һ |
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ_______ɫ | ������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | _______________________________ |
| Ȼ����Һ����ɫ��Ϊ_______ɫ | _______________________________ |
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
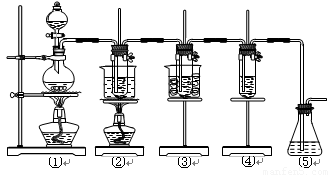
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
(A)��ʯ�� (B)����ʳ��ˮ (C)Ũ���� (D)����̼��������Һ
(2)�Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�____________________________��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ_______ɫ |
������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
|
�����Һ��Ϊ��ɫ |
_______________________________ |
|
Ȼ����Һ����ɫ��Ϊ_______ɫ |
_______________________________ |