��Ŀ����
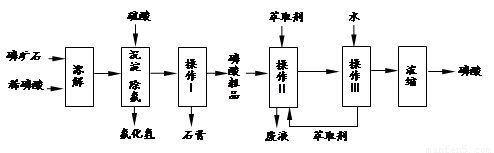
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
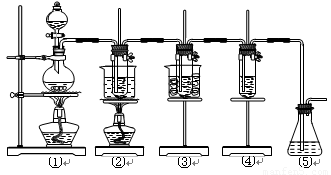
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
(A)��ʯ�� (B)����ʳ��ˮ (C)Ũ���� (D)����̼��������Һ
(2)�Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�____________________________��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ_______ɫ |
������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
|
�����Һ��Ϊ��ɫ |
_______________________________ |
|
Ȼ����Һ����ɫ��Ϊ_______ɫ |
_______________________________ |
(1)��Һ©����B��
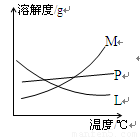
(2)����Һ(��Ӧ��)��Ũ�Ȳ�ͬ����Ӧ�¶Ȳ�ͬ�� M�� ���ˡ�
(3)Cl2��2OH�� = ClO����Cl����H2O��
(4)
|
�� |
|
|
|
������ˮ��Ӧ����HClO��ʯ������Ϊ��ɫ���� |
|
���� |
����ͨ�����������ˮʹ��Һ�ʻ���ɫ |
����������

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
(A)��ʯ�� (B)����ʳ��ˮ (C)Ũ���� (D)����̼��������Һ
(2)�Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�____________________________��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ_______ɫ | ������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | _______________________________ |
| Ȼ����Һ����ɫ��Ϊ_______ɫ | _______________________________ |
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
| A����ʯ�� | B������ʳ��ˮ | C��Ũ���� | D������̼��������Һ |
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��

(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ_______ɫ | ������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | _______________________________ |
| Ȼ����Һ����ɫ��Ϊ_______ɫ | _______________________________ |