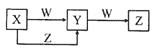
��Ŀ����
����Ŀ����ͼ����ѧ��ѧ�г������ʼ��ת����ϵ�����мס��ҡ�����Ϊ�ǽ������ʣ�A��B��E�Ͷ���Ϊ�����BΪ�������ܲ�������ЧӦ�����壻E������л���ұ�״�����ܶ�Ϊ0.714g��L-1���ҺͶ�Ϊ��ɫ���壬�����ǻ�Ϻ���ȷ��ֹ����ɺڱ�졣
��1��д����ѧʽ����___����___��
��2������Ħ������__��
��3��д������E��Ӧ����A��B�Ļ�ѧ����ʽ___��
��4����ͬѧ���ҺͶ���ϼ��Ⱥ��ռ����ı�״̬������8.96L����ø������������ܶȵ�16������������ͨ����������ʯ��ˮ���õ���ɫ������___g��
���𰸡�C O2 80g/mol CH4+2O2![]() CO2+2H2O 10
CO2+2H2O 10
��������
BΪ�������ܲ�������ЧӦ�����壬��ΪCO2��E������л���ұ�״�����ܶ�Ϊ0.714g��L-1����֪��Ħ������Ϊ0.714g��L-1��22.4L/mol=16g/mol����EΪ����(CH4)���ҺͶ�Ϊ��ɫ��������Ϊ�ǽ������ʣ���֪��ΪC���ҺͶ���Ϻ���ȷ��ֹ����ɺڱ�죬˵����ΪCuO���ס��ҡ�����Ϊ�ǽ������ʣ���˽�һ����ȷ����ΪO2����ΪH2���ݴ˷�������
��1���ɷ�����֪��ΪC����ΪO2��
��2���ɷ�����֪��ΪCuO����Ħ������Ϊ80g/mol��
��3���ɷ�����֪��ΪO2��EΪ���飬��������������ȫȼ�գ�����CO2��ˮ����Ӧ����ʽΪ��CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
��4����ΪC����ΪCuO����ϼ��ȷ���������ԭ��Ӧ����ø������������ܶȵ�16�������õ��������Ħ������Ϊ16��2g/mol=32g/mol����28<32<44����֪���ɵ�������CO2��CO�Ļ�����壬����������CO2�����ʵ���Ϊxmol����ô��![]() =32�����x=0.1mol������Cԭ���غ��֪n(CaCO3)=n(CO2)=0.1mol����˰�ɫ����������Ϊ0.1mol��100g/mol=10g��
=32�����x=0.1mol������Cԭ���غ��֪n(CaCO3)=n(CO2)=0.1mol����˰�ɫ����������Ϊ0.1mol��100g/mol=10g��

 ��У����ϵ�д�
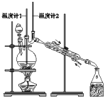
��У����ϵ�д�����Ŀ����֪��CH3CH2CH2CH2OH![]() CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
���� | �е�/�� | �ܶ� / gcm-3 | ˮ���ܽ��� |
|
������ | 117.2 | 0.8109 | �� | |
����ȩ | 75.7 | 0.8017 | �� |
����˵���У�����ȷ����
A.Ϊ��ֹ�����һ��������Ӧ���ữ��Na2Cr2O7��Һ��μ�����������
B.���¶ȼ�1ʾ��Ϊ90~95�����¶ȼ�2ʾ����76������ʱ���ռ�����
C.��Ӧ������������ﵹ���Һ©���У���ȥˮ�㣬������ȩ�ӷ�Һ©���Ͽڵ���
D.���õĴ�����ȩ�м������������ƣ����������Ƿ���������
����Ŀ���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������60mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺

(1)��ͼ��ʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��_______������֮�⣬װ���е�һ�����Դ�����______��
(2)Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������______��
(3)������60mL0.25mol��L-1H2SO4��60mL0.55mol��L-1NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������____����������������������������ʵ���������ȷ���������к���_____��������������������
(4)����NaOH��Һ����ȷ�����ǣ�______��(������ѡ��)��
A.�ز������������� B.�������������� C.һ��Ѹ�ٵ���
(5)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�______�� (������ѡ��)��
A.���¶ȼ�С�Ľ��� B.�ҿ�ӲֽƬ�ò���������
C.��������ձ� D.�������¶ȼ��ϵĻ��β���������ؽ���
(6)ʵ���������±���
������д�±��еĿհף�
�¶� ʵ�� ���� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ______ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55mol/LNaOH��Һ��0.25mol/L������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c=4.18J/(g����)�����к�����H=________(ȡС�����һλ)��
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)______��
a.ʵ��װ�ñ��¡�����Ч����
b.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���