��Ŀ����
����10�֣��ش������й����ڱ������⣺
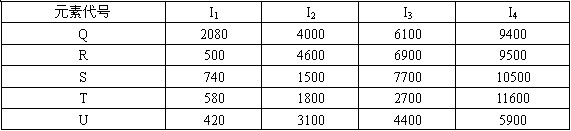
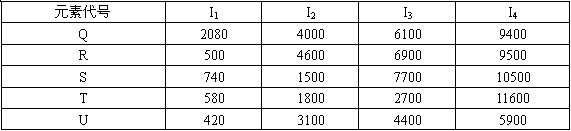
��1������ͼ��ʾԪ�����ڱ�������ʵ�ߣ���ɫ���Աʣ���������Ԫ�صı߽硣
��2��Ԫ�ؼ��ǵ������ڢ�A��Ԫ�أ���Ԫ���������ַǽ���Ԫ�ؿ�������ӻ�����A��д��A�ĵ���ʽ________��
��3�������е���̬�⻯���У�Ԫ���ҵ��⻯�����ȶ���д��Ԫ���ҵĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ_____________________________________________________________
��4������Ԫ�ر��γɵ�ij���������Һ�����ڼ���CO2����Ԫ�ر��ļ�������Ԫ�ؼļ����ӵİ뾶��С��ϵ��________________(�����ӷ��ű�ʾ)
��5��������ֱ��ǵ��������м����Ӱ뾶��С�����Ľ���Ԫ�أ�д����Ԫ�صĵ�����������������Ӧ��ˮ������Һ��Ӧ�����ӷ���ʽ_____________________��
������ʽ2�֣���10�֣�
(1) 
(2) 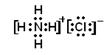 ����(3)2F2��2H2O===4HF��O2
����(3)2F2��2H2O===4HF��O2
(4)r(Ca2��)<r(Cl��)����(5)��2Al��2OH-��2H2O��2AlO2-��3H2��
����

��ϰ��ϵ�д�
 ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ