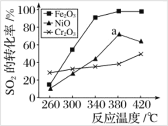
��Ŀ����
����Ŀ����[aX��bY]Ϊ a �� X ���� b �� Y ����ɵ�һ���������壬N(z)Ϊ�� z ��������NA Ϊ�����ӵ�������ֵ������˵��һ������ȷ����
A.C(ʯī)��O2(g) = CO2(g) ��H����390 kJ��mol1����ÿ 1 mol [![]() C(ʯī)��
C(ʯī)��![]() O2(g)]��ȫȼ�շ��� 130 kJ
O2(g)]��ȫȼ�շ��� 130 kJ
B.Cu �� 1mol/L ��������Һ��ַ�Ӧ�������� 22.4 L ���壬��ת�Ƶ�����Ϊ 3NA
C.��״���� 1.6g ������������Ϊ 0.05NA����ƽ��ÿ�� O2 ���ӵ����ԼΪ ![]() L
L
D.1 mol Cl2 ��ϡ NaOH ��Һ��ȫ��Ӧ����Ӧ�����Һ�� N(ClO)��N(HClO)��1NA
���𰸡�B
��������
A. ����1molʯī��1molO2��Ӧ�ų�390 kJ�������������ÿ 1 mol [![]() C(ʯī)��
C(ʯī)��![]() O2(g)]��ȫȼ�շų���������
O2(g)]��ȫȼ�շų���������
B. �����δ������Ӧ�����������������м��㣻
C. 1.6g ���������ʵ���Ϊ![]() =0.05mol��������Ϊ0.05NA����״���������Ϊ0.05mol��22.4L/mol=1.12L���ݴ˼���ÿ�� O2 ���ӵ������
=0.05mol��������Ϊ0.05NA����״���������Ϊ0.05mol��22.4L/mol=1.12L���ݴ˼���ÿ�� O2 ���ӵ������
D. 1 mol Cl2 ��ϡ NaOH ��Һ��ȫ��Ӧ������NaCl��NaClO��ClO-ˮ������HClO���ݴ˼���N(ClO)��N(HClO)��
A. 1molʯī��1molO2��Ӧ�ų�390 kJ�����������ÿ 1 mol [![]() C(ʯī)��
C(ʯī)��![]() O2(g)]��ȫȼ�շų�������Ϊ
O2(g)]��ȫȼ�շų�������Ϊ![]() ��390 kJ=130 kJ��A����ȷ�����������⣻
��390 kJ=130 kJ��A����ȷ�����������⣻
B. �����δ������Ӧ�����������Ƿ�Ϊ��״������������м��㣬B����������⣻
C. 1.6g ���������ʵ���Ϊ![]() =0.05mol��������Ϊ0.05NA����״���������Ϊ0.05mol��22.4L/mol=1.12L�����ÿ�� O2 ���ӵ����Ϊ
=0.05mol��������Ϊ0.05NA����״���������Ϊ0.05mol��22.4L/mol=1.12L�����ÿ�� O2 ���ӵ����Ϊ![]() L��C����ȷ�����������⣻
L��C����ȷ�����������⣻
D. 1 mol Cl2 ��ϡ NaOH ��Һ��ȫ��Ӧ������NaCl��NaClO��ClO-ˮ������HClO�����N(ClO)��N(HClO)= 1NA��D����ȷ�����������⣻
��ѡB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�