��Ŀ����
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ��������ͼ��ʾ��
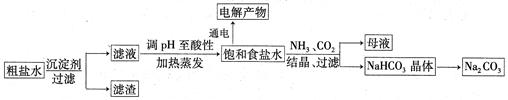
��1��Ϊ��ȥ�����е�Ca2+��Mg2+��SO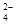 ���õ�������NaCl���壬����������Լ���
���õ�������NaCl���壬����������Լ���
��ȷ�ļ���˳����_______����ȱ�ٵIJ���������__________��___________��
��2������Һ��pH�������Գ�ȥ��������________��������Ӧ�����ӷ���ʽΪ________��
��3�����ö��Ե缫���200mL1.5mol/Lʳ��ˮ�������2minʱ���������ռ���448mL���壨��״���£���������ǰ����Һ��������䣬��������Һ��pHΪ______��
��4����������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ��___________��
��5�������������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۡ���ԭ���ǣ�������ӷ���ʽ������_____________��
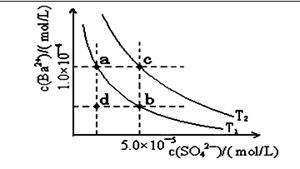
�ڳ����£���ijpH=11��Na2CO3��Һ�м������ʯ���飬���˺�������ҺpH=13����Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������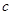 ��OH-���ı�ֵ��_________��
��OH-���ı�ֵ��_________��

��1��Ϊ��ȥ�����е�Ca2+��Mg2+��SO
 ���õ�������NaCl���壬����������Լ���
���õ�������NaCl���壬����������Լ���| A��������NaOH��Һ�� | B��������Na2CO3��Һ�� | C����������� | D��������BaCl2��Һ�� |
��2������Һ��pH�������Գ�ȥ��������________��������Ӧ�����ӷ���ʽΪ________��
��3�����ö��Ե缫���200mL1.5mol/Lʳ��ˮ�������2minʱ���������ռ���448mL���壨��״���£���������ǰ����Һ��������䣬��������Һ��pHΪ______��
��4����������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ��___________��
��5�������������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۡ���ԭ���ǣ�������ӷ���ʽ������_____________��
�ڳ����£���ijpH=11��Na2CO3��Һ�м������ʯ���飬���˺�������ҺpH=13����Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������
 ��OH-���ı�ֵ��_________��
��OH-���ı�ֵ��_________����1�� adbc��dabc (2��) ���˺������ᾧ (2��)
��2��OH-��CO32- (2��) H+ + OH- = H2O (1��) CO32- + 2H+ = CO2��+ H2O (1��)
��3��13 (2��)
��4��NH3 (1��)
 ��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
��2��OH-��CO32- (2��) H+ + OH- = H2O (1��) CO32- + 2H+ = CO2��+ H2O (1��)
��3��13 (2��)
��4��NH3 (1��)
 ��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)�����������1�����ӹ����У���ʱΪ�ܽ�����ȫ����ȥ�����ó����Լ������Լӹ��������������Լ�����Ҫ�ܱ������Լ��������������˽����ʵ�鲽���Ŀ�ģ����ܷ������˳���������NaOH��Һ��Ŀ���dz�ȥMg2�������������BaCl2��Һ��Ϊ�˳�ȥSO42-�����������Na2CO3��Һ��Ϊ�˳�ȥCa2���������Ba2����Ȼ����й��ˣ���ȥMg(OH)2��BaSO4��CaCO3��BaCO3�������Ȼ������������ᣬ��ȥ�����Na2CO3�͵�����Һ��pH��������������ȷ��ʵ�����˳��Ϊadbc��dabc����ȱ�ٵIJ��������ǹ��˺������ᾧ��
��2��������NaOH��Na2CO3��Ӧ�����ӷ���ʽΪ��H+ + OH- = H2O��CO32- + 2H+ = CO2��+ H2O��
��3�������Ȼ�����Һ��Ӧ�����ӷ���ʽΪ��2Cl?+2H2O
 2OH?+H2��+Cl2�������ܷ�Ӧ�������Ϲ��ռ���0.448L��0.02 mol����ʱ�������ɵ���������������0.01mol�����������������Ƶ����ʵ�����0.02mol�������������Ƶ�Ũ��C=0.02mol��0.2L=0.1mol?L?1,����pH=13��
2OH?+H2��+Cl2�������ܷ�Ӧ�������Ϲ��ռ���0.448L��0.02 mol����ʱ�������ɵ���������������0.01mol�����������������Ƶ����ʵ�����0.02mol�������������Ƶ�Ũ��C=0.02mol��0.2L=0.1mol?L?1,����pH=13����4�������NaHCO3������ĸҺ��NH4Cl�����������ʯ�ң�NH4Cl��Ca(OH)2��Ӧ��õĿ���ѭ��ʹ�õ�����ΪNH3��
��5����Na2CO3Ϊǿ�������Σ�ˮ���Լ��ԣ����ӷ���ʽΪ��CO32- + H2O
 HCO3- + OH-��������Һ�ɳ����ۡ�
HCO3- + OH-��������Һ�ɳ����ۡ��ڷ�Ӧǰ����Һ��ˮ�������c��OH-��=1.0��10-14��10-11=1.0��10-3mol/L����Ӧ����ҺΪpH=13��ˮ�������c��OH-��=1.0��10-13mol/L�����Ա�ֵΪ1.0��10-3mol?L?1��1.0��10-13mol?L?1=1010��1��

��ϰ��ϵ�д�
�����Ŀ
 ����pH��ֽ���M��Һ��pH��ֽ����ɫ
����pH��ֽ���M��Һ��pH��ֽ����ɫ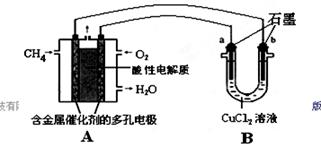
 CH3COO-��H+
CH3COO-��H+ C6H5O-��H+
C6H5O-��H+ NH4+��OH-
NH4+��OH-