��Ŀ����
��10�֣��Ͼ�ӡˢ��·��Ļ������ÿ�ʵ������������������Ⱦ���Ͼ�ӡˢ��·�徭������룬�ܵõ��ǽ�����ĩ�ͽ�����ĩ��
��1����2�֣����д���ӡˢ��·��ǽ�����ĩ�ķ����У������ϻ�������������� ������ĸ����
| A�����ѽ��γ�ȼ�� | B��¶����� |
| C����Ϊ�л����Ͻ������ϵ�ԭ�� | D��ֱ������ |
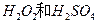 �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��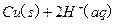 ====
====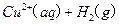
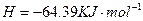
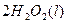 ====
====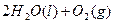
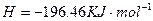
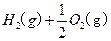 ="==="
="===" 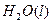
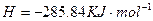
��
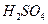 ��Һ��
��Һ��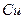 ��
��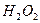 ��Ӧ����
��Ӧ����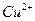 ��
��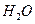 ���Ȼ�����ʽΪ ��
���Ȼ�����ʽΪ ����3����3�֣���������������ͬ��ӡˢ��·��Ľ�����ĩ��10�G
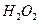 ��3.0
��3.0 �Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����� �� 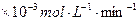 ��[:.....] ��[:.....] | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
��4����3�֣����ᴿ���
 ��Һ�м���һ������
��Һ�м���һ������ ��
�� ��Һ�����ȣ�����
��Һ�����ȣ����� �������Ʊ�
�������Ʊ� �����ӷ���ʽ�� ��
�����ӷ���ʽ�� ��
��1��BD
��2��Cu��S��+H2O2��l��+2H+��aq��=====Cu2+(aq)+2H20��l����H=-319.68J��mol-1
��3��H2O2�ֽ����ʼӿ�
��4��2Cu2++SO32-+2Cl-+H2O 2CuCl ��+SO42-+2H+
2CuCl ��+SO42-+2H+
����

 ������ϵ�д�
������ϵ�д�(1)���д���ӡˢ��·��ǽ�����ĩ�ķ����У������ϻ��������������_______(����ĸ)��
A.���ѽ��γ�ȼ��
B.¶�����
C.��Ϊ�л����Ͻ������ϵ�ԭ��
D.ֱ������
(2)��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
Cu(s)+2H+ (aq)====Cu2+ (aq)+H2(g) ��H��64.39 kJ��mol-1
2H2O2(l)====2H2O(l)+O2(g) ��H��-196.46 kJ��mol-1
![]() ====H2O(l) ��H��-285.84 kJ��mol-1
====H2O(l) ��H��-285.84 kJ��mol-1
��H2SO4��Һ��Cu��H2O2��Ӧ����Cu2+��H2O���Ȼ�ѧ����ʽΪ_____________________��
(3)��������������ͬ��ӡˢ��·��Ľ�����ĩ��10%H2O2��3.0 mol��L-1 H2SO4�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ�����(���±�)��
�¶�/�� | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
ͭƽ���ܽ�����/��10-3 mol��L-1��min-1 | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
���¶ȸ���
(4)���ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ��_________________________________________________________��
�Ͼ�ӡˢ��·��Ļ������ÿ�ʵ����Դ��������������Ⱦ���Ͼ�ӡˢ��·�徭������룬�ܵõ��ǽ�����ĩ�ͽ�����ĩ��
��1�����д���ӡˢ��·��ǽ�����ĩ�ķ����У������ϻ�������������� ������ĸ����
A�����ѽ��γ�ȼ��B��¶����� C����Ϊ�л����Ͻ������ϵ�ԭ��D��ֱ������
��2����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
Cu(s)��2H��(aq)=Cu2��(aq)��H2(g) ��H=64.39kJ��mol��1
2H2O2(l)=2H2O(l)��O2(g) ��H=��196.46kJ��mol��1
H2(g)��1/2O2(g)=H2O(l)
��H=��285.84kJ��mol��1
�� H2SO4��Һ��Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ ��
��3����������������ͬ��ӡˢ��·��Ľ�����ĩ��10�GH2O2��3.0mol��L��1H2SO4�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
|
�¶ȣ��棩 |
20 |
30 |
40 |
50 |
60 |
70 |
80 |
|
ͭƽ���ܽ����ʣ���10-3 mol��L-1��min-1�� |
7.34 |
8.01 |
9.25 |
7.98 |
7.24 |
6.73 |
5.76 |
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
��4�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
��5�� ��֪��ͬ�����£�
4Ca5(PO4)3F(s)+3SiO2(s)=6Ca3(PO4)2(s)+2CaSiO3(s)+SiF4(g) ����H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g)����H2
SiO2(s)+CaO(s)=CaSiO3(s) ����H3
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) �� H
H
�á�H1����H2�͡�H3��ʾ H��
H�� H=
��
H=
��
��6����֪1 g FeS2(s)��ȫȼ�����ɷų�7.1 kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ ��
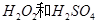 �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ�� ��֪��
��֪��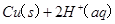 ====
====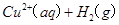
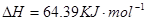
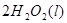 ====
====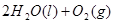
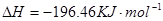
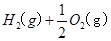 ====
====
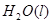

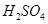 ��Һ��
��Һ��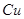 ��
��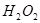 ��Ӧ����
��Ӧ����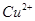 ��
��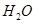 ���Ȼ�ѧ����ʽΪ
���Ȼ�ѧ����ʽΪ