ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ”–ΜζΈοA≥Θ”Ο”Ύ ≥ΤΖ––“ΒΓΘ“―÷Σ9.0 g A‘ΎΉψΝΩO2÷–≥δΖ÷»Φ…’Θ§ΫΪ…ζ≥…ΒΡΜλΚœΤχΧε“ά¥ΈΆ®ΙΐΉψΝΩΒΡ≈®ΝρΥαΚΆΦν ·Μ“Θ§Ζ÷±π‘ω÷Ί5.4gΚΆ13.2 gΘ§Ψ≠Φλ―ι Θ”ύΤχΧεΈΣO2ΓΘ

Θ®1Θ© AΖ÷Ή”ΒΡ÷ ΤΉΆΦ»γΆΦΥυ ΨΘ§¥”ΆΦ÷–Ω…÷ΣΤδœύΕ‘Ζ÷Ή”÷ ΝΩ «___________Θ§‘ρAΒΡΖ÷Ή” Ϋ «___________ΘΜ
Θ®2Θ©AΡή”κNaHCO3»ή“ΚΖΔ…ζΖ¥”ΠΘ§A“ΜΕ®Κ§”–ΒΡΙΌΡήΆ≈Οϊ≥Τ «___________ΓΘ
Θ®3Θ©AΖ÷Ή”ΒΡΚΥ¥≈Ι≤’ώ«βΤΉ”–4ΗωΈϋ ’ΖεΘ§ΖεΟφΜΐ÷°±» «1ΘΚ1ΘΚ1ΘΚ3Θ§‘ρAΒΡΫαΙΙΦρ Ϋ «___________ΘΜ
Θ®4Θ©–¥≥ωΝΫΖ÷Ή”A…ζ≥…Νυ‘ΣΜΖθΞΒΡΜ·―ßΖΫ≥Χ ΫΘΚ ΘΜ
Θ®5Θ©«κ–¥≥ωΙΌΡήΆ≈”κAœύΆ§ΒΡΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ Ϋ___________ΓΘ
ΓΨ¥πΑΗΓΩ
Θ®1Θ©90ΘΜC3H6O3
Θ®2Θ©τ»Μυ
Θ®3Θ©CH3CHΘ®OHΘ©COOH
Θ®4Θ©2CH3CH(OH)COOH![]()
 +2H2O
+2H2O
Θ®5Θ©HOCH2CH2COOH
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΘ®1Θ©5.4gΥ°ΒΡΈο÷ ΒΡΝΩΈΣ![]() =0.3molΘ§n(H)=0.6molΘ§13.2gΕΰ―θΜ·ΧΦΒΡΈο÷ ΒΡΝΩΈΣ
=0.3molΘ§n(H)=0.6molΘ§13.2gΕΰ―θΜ·ΧΦΒΡΈο÷ ΒΡΝΩΈΣ![]() =0.3molΘ§n(C)=n(CO2)=0.3molΘ§¥Υ”–ΜζΈο9.0gΚ§O‘ΣΥΊ÷ ΝΩΘΚ9.0g-0.6g-0.3ΓΝ12 g=4.8 gΘ§n(O)=
=0.3molΘ§n(C)=n(CO2)=0.3molΘ§¥Υ”–ΜζΈο9.0gΚ§O‘ΣΥΊ÷ ΝΩΘΚ9.0g-0.6g-0.3ΓΝ12 g=4.8 gΘ§n(O)= ![]() =0.3molΘ§Ι n(C)ΘΚn(H)ΘΚn(O)=0.3molΘΚ0.6molΘΚ0.3mol=1ΘΚ2ΘΚ1Θ§Φ¥ Β―ι ΫΈΣCH2OΘ§…ηΖ÷Ή” ΫΈΣ(CH2O)nΘ§ΗυΨίΤΉΆΦ»γΆΦΥυ Ψ÷ Κ…±»Θ§Ω…÷ΣAΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ90Θ§Ω…ΒΟ30n=90Θ§ΫβΒΟΘΚn=3Θ§Ι ”–ΜζΈοAΈΣC3H6O3ΓΘ
=0.3molΘ§Ι n(C)ΘΚn(H)ΘΚn(O)=0.3molΘΚ0.6molΘΚ0.3mol=1ΘΚ2ΘΚ1Θ§Φ¥ Β―ι ΫΈΣCH2OΘ§…ηΖ÷Ή” ΫΈΣ(CH2O)nΘ§ΗυΨίΤΉΆΦ»γΆΦΥυ Ψ÷ Κ…±»Θ§Ω…÷ΣAΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ90Θ§Ω…ΒΟ30n=90Θ§ΫβΒΟΘΚn=3Θ§Ι ”–ΜζΈοAΈΣC3H6O3ΓΘ
Ι ¥πΑΗΈΣΘΚ90ΘΜC3H6O3ΘΜ
Θ®2Θ©AΡή”κNaHCO3»ή“ΚΖΔ…ζΖ¥”ΠΘ§A“ΜΕ®Κ§”–τ»ΜυΘ®-COOHΘ©ΓΘ
Ι ¥πΑΗΈΣΘΚτ»ΜυΘΜ
Θ®3Θ©”–ΜζΈοAΈΣC3H6O3Θ§ΚΥ¥≈Ι≤’ώ«βΤΉ”–4ΗωΖεΘ§ΖεΟφΜΐ÷°±» «1ΘΚ1ΘΚ1ΘΚ3Θ§‘ρΖ÷Ή”÷–4÷÷H‘≠Ή”ΒΡ
ΐΡΩΈΣ1ΓΔ1ΓΔ1ΓΔ3Θ§Ζ÷Ή”÷–Κ§”–1Ηω-COOHΓΔ1Ηω-CH3ΓΔ1Ηω![]() ΓΔ1Ηω-OHΘ§”–ΜζΈοAΒΡΫαΙΙΦρ ΫΈΣCH3CH(OH)COOHΓΘ
ΓΔ1Ηω-OHΘ§”–ΜζΈοAΒΡΫαΙΙΦρ ΫΈΣCH3CH(OH)COOHΓΘ
Ι ¥πΑΗΈΣΘΚCH3CH(OH)COOHΘΜ
Θ®4Θ©ΝΫΖ÷Ή”»ιΥαCH3CH(OH)COOH‘Ύ“ΜΕ®ΧθΦΰœ¬Ω…ΖΔ…ζθΞΜ·Ζ¥”Π…ζ≥…Νυ‘ΣΜΖΉ¥Μ·ΚœΈοΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣΘΚ
2CH3CH(OH)COOH![]()
 +2H2OΓΘ
+2H2OΓΘ
Ι ¥πΑΗΈΣΘΚ2CH3CH(OH)COOH![]()
 +2H2OΘΜ
+2H2OΘΜ
Θ®5Θ©ΙΌΡήΆ≈”κAœύΆ§ΒΡΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΈΣΘΚHOCH2CH2COOHΓΘ
Ι ¥πΑΗΈΣΘΚHOCH2CH2COOHΓΘ

 Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
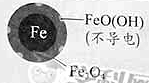
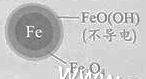
Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ”ΟΝψΦέΧζΘ®FeΘ©»Ξ≥ΐΥ°Χε÷–ΒΡœθΥα―ΈΘ®NO3-Θ©“―≥…ΈΣΜΖΨ≥–όΗ¥―–ΨΩΒΡ»»Βψ÷°“ΜΓΘ
Θ®1Θ©FeΜΙ‘≠Υ°Χε÷–NO3-ΒΡΖ¥”Π‘≠άμ»γΆΦΥυ ΨΓΘ

ΔΌΉςΗΚΦΪΒΡΈο÷ «________ΓΘ
ΔΎ’ΐΦΪΒΡΒγΦΪΖ¥”Π Ϋ «_________ΓΘ
Θ®2Θ©ΫΪΉψΝΩΧζΖέΆΕ»κΥ°Χε÷–Θ§Ψ≠24–Γ ±≤βΕ®NO3ΓΣΒΡ»Ξ≥ΐ¬ ΚΆpHΘ§ΫαΙϊ»γœ¬ΘΚ
≥θ ΦpH | pH=2.5 | pH=4.5 |
NO3ΓΣΒΡ»Ξ≥ΐ¬ | Ϋ”Ϋϋ100% | ΘΦ50% |
24–Γ ±pH | Ϋ”Ϋϋ÷––‘ | Ϋ”Ϋϋ÷––‘ |
ΧζΒΡΉν÷’Έο÷ –ΈΧ§ |
|
|
pH=4.5 ±Θ§NO3ΓΣΒΡ»Ξ≥ΐ¬ ΒΆΓΘΤδ‘≠“ρ «________ΓΘ
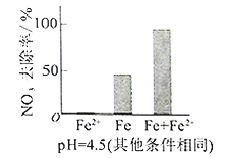
Θ®3Θ© Β―ιΖΔœ÷ΘΚ‘Ύ≥θ ΦpH=4.5ΒΡΥ°Χε÷–ΆΕ»κΉψΝΩΧζΖέΒΡΆ§ ±Θ§≤Ι≥δ“ΜΕ®ΝΩΒΡFe2+Ω…“‘Οςœ‘ΧαΗΏNO3ΓΣΒΡ»Ξ≥ΐ¬ ΓΘΕ‘Fe2+ΒΡΉς”ΟΧα≥ωΝΫ÷÷ΦΌ…ηΘΚ
Δώ. Fe2+÷±Ϋ”ΜΙ‘≠NO3ΓΣΘΜ
Δρ. Fe2+ΤΤΜΒFeOΘ®OHΘ©―θΜ·≤ψΓΘ
ΔΌΉωΕ‘±» Β―ιΘ§ΫαΙϊ»γΆΦΥυ ΨΘ§Ω…ΒΟΒΫΒΡΫα¬έ «_______ΓΘ
ΔΎΆ§ΈΜΥΊ ΨΉΌΖ®÷Λ ΒFe2+Ρή”κFeOΘ®OHΘ©Ζ¥”Π…ζ≥…Fe3O4ΓΘΫαΚœΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘ§Ϋβ ΆΦ”»κFe2+ΧαΗΏNO3ΓΣ»Ξ≥ΐ¬ ΒΡ‘≠“ρΘΚ______ΓΘ
Θ®4Θ©ΤδΥϊΧθΦΰ”κΘ®2Θ©œύΆ§Θ§Ψ≠1–Γ ±≤βΕ®NO3ΓΣΒΡ»Ξ≥ΐ¬ ΚΆpHΘ§ΫαΙϊ»γœ¬ΘΚ
≥θ ΦpH | pH=2.5 | pH=4.5 |
NO3ΓΣΒΡ»Ξ≥ΐ¬ | ‘Φ10% | ‘Φ3% |
1–Γ ±pH | Ϋ”Ϋϋ÷––‘ | Ϋ”Ϋϋ÷––‘ |
”κΘ®2Θ©÷– ΐΨίΕ‘±»Θ§Ϋβ ΆΘ®2Θ©÷–≥θ ΦpH≤ΜΆ§ ±Θ§NO3ΓΣ»Ξ≥ΐ¬ ΚΆΧζΒΡΉν÷’Έο÷ –ΈΧ§≤ΜΆ§ΒΡ‘≠“ρΘΚ__________ΓΘ
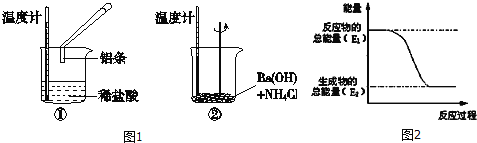
ΓΨΧβΡΩΓΩœ¬Ν–―Γœν÷–ΡήΖΔ…ζΜ·―ßΖ¥”ΠΘ§«“ΦΉΉιΈΣ»Γ¥ζΖ¥”ΠΓΔ““ΉιΈΣΦ”≥…Ζ¥”ΠΒΡ «Θ® Θ©
ΦΉ | ““ | |
A | ±Ϋ”κδεΥ° | ““œ©”κΥ°÷Τ““¥ΦΘ®¥ΏΜ·ΦΝΘ© |
B | ”Ά÷§Υ°Ϋβ | ±Ϋ”κ«βΤχΘ®¥ΏΜ·ΦΝΓΔΦ”»»Θ© |
C | ΦΉΆι”ꬻΤχΘ®‘ΎΙβΝΝ¥ΠΘ© | ““ΥαΚΆ““¥ΦΒΡθΞΜ·Ζ¥”ΠΘ®¥ΏΜ·ΦΝΓΔΦ”»»Θ© |
D | ““œ©”κδεΒΡΥΡ¬»Μ·ΧΦ»ή“Κ | ““¥Φ”κΡΤΖ¥”Π |