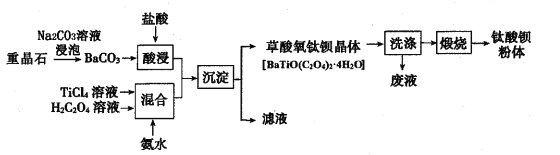
��Ŀ����
����Ŀ��һ���¶��£���ij�ܱ������з�����Ӧ��2HI(g)![]() H2(g)��I2(g)����H>0����15s��c(HI)��0.1mol��L��1����0.07mol��L��1��������˵����ȷ����(��)
H2(g)��I2(g)����H>0����15s��c(HI)��0.1mol��L��1����0.07mol��L��1��������˵����ȷ����(��)
A. 0��15 s����I2��ʾ��ƽ����Ӧ����Ϊv(I2)��0.002 mol��L��1��s��1
B. c(HI)��0.07 mol��L��1����0.05 mol��L��1����ķ�Ӧʱ��С��10 s
C. �����¶�����Ӧ���ʼӿ죬�淴Ӧ���ʼ���
D. ��С��Ӧ��ϵ���������ѧ��Ӧ���ʼӿ�
���𰸡�D
��������A���������ù����ʾ��ѧ��Ӧ���ʣ�A����B����c��HI����0.1mol/L ����0.07mol/Lʱ����Ҫ15s��������0.03mol/L��Ҫ15s��c��HI����0.07mol/L ����0.05mol/Lʱ��Ũ�ȼ���0.02mol/L����������Ũ�ȱ仯������ʱ����Ҫ0.02mol/L/0.03mol/L��15s=10s����Ũ��ԽС����ѧ��Ӧ����ԽС����Ҫ��ʱ��ͳ���������Ҫʱ�����10s��B����C�������κλ�ѧ��Ӧ�������¶ȣ����淴Ӧ���ʾ��ӿ죬C����D���÷�ӦΪ�����������ķ�Ӧ����С�����������ѹǿ����ѧ��Ӧ���ʼӿ죬D��ȷ����ѡD��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ