ћвƒњƒЏ»Ё
ЌйћюA÷їњ…ƒ№”–»э÷÷“ї¬»»°іъ≤ъќпB°ҐCЇЌD.CµƒљбєєЉт љ «![]() °£BЇЌDЈ÷±р”л«њЉоµƒіЉ»№“Їє≤»»£ђґЉ÷їƒ№µ√µљ”–їъїѓЇѕќпE.“‘…ѕЈі”¶Љ∞Bµƒљш“ї≤љЈі”¶»зѕ¬ЌЉЋщ Њ.
°£BЇЌDЈ÷±р”л«њЉоµƒіЉ»№“Їє≤»»£ђґЉ÷їƒ№µ√µљ”–їъїѓЇѕќпE.“‘…ѕЈі”¶Љ∞Bµƒљш“ї≤љЈі”¶»зѕ¬ЌЉЋщ Њ.
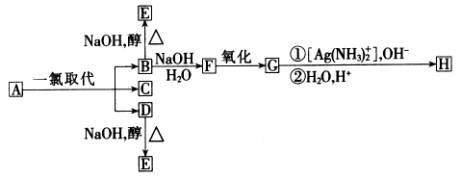
«лїЎір£Ї
(1)AµƒљбєєЉт љ «__________°°°° ______.
(2)HµƒљбєєЉт љ «_________°°°°°°°° _______.
(3)B„™±дќ™FµƒЈі”¶ ф”Џ____ ____Јі”¶(ћоЈі”¶ја–Ќ√ы≥∆).
(4)B„™±дќ™EµƒЈі”¶ ф”Џ_____ ___Јі”¶(ћоЈі”¶ја–Ќ√ы≥∆).
(5)1.16gH”л„гЅњNaHCO3„ч”√£ђ±књцѕ¬њ…µ√CO2µƒћеїэ «________mL.
љвќц£Ї
(1) (CH3)3CCH2CH3 (2)(CH3)3CCH2COOH (3)»°іъ(ір¬»іъћюЋЃљв“≤ЄшЈ÷) (4)ѕы»• (5)224
|
ћб Њ£Ї
±Њћв“‘”–їъњтЌЉµƒ–ќ љ≥цѕ÷.њЉ≤йЅЋЌђЈ÷“мєєће°Ґ”–їъЈ÷„”љбєєµƒ»Јґ®°Ґ”–їъЈі”¶°ҐЈі”¶ја–ЌЉ∞ѕаєЎЉ∆Ћгµ»÷™ ґ.љвћв≤љ÷и»зѕ¬£Ї (1)ЄщЊЁЌйћюAµƒ“ї¬»»°іъ≤ъќпCµƒљбєєЉт љ (2)B°ҐDЅљ÷÷“ї¬»»°іъ≤ъќпЈҐ…ъѕы»•Јі”¶µƒ≤ъќпЊщќ™ (3)ЄщЊЁЈљ≥ћ љЉ∆Ћг≥цCO2µƒћеїэ£ђєэ≥ћ»зѕ¬£Ї (CH3)3CCH2COOH£ЂNaHCO3°ъ (CH3)3CCH2COONa£ЂCO2°ь£ЂH2O 116g°°°°°°°°°°°°°°°° 22.4L 1.16g°°°°°°°°°°°°°°°° x 116g:1.16g=22.4L:x ”–їъЈ÷„”љбєєµƒ»Јґ®Љ∞”–їъЈі”¶ «њЉЇЋµƒ÷Ўµг£ђіЋћвќіЄш≥ц»ќЇќ й±Њ“‘Ќвµƒ–¬–≈ѕҐ£ђ“тіЋ”¶„Ґ÷Ўїщі°÷™ ґµƒ—Іѕ∞”лЈҐ…Ґ.іЋћв…жЉ∞ЅЋ»эЄц÷™ ґƒ—µг£Ї“ї «µ»–ІH‘≠„”µƒ—∞’“£ђAµƒљбєєЉт љќ™
÷ї”–
£їґш
|

 √ҐєыљћЄ®іп±к≤в ‘ЊнѕµЅ–ір∞Є
√ҐєыљћЄ®іп±к≤в ‘ЊнѕµЅ–ір∞Є £ђ»Јґ®AµƒљбєєЉт љќ™
£ђ»Јґ®AµƒљбєєЉт љќ™ £ђЅнЅљ÷÷“ї¬»»°іъ≤ъќпќ™
£ђЅнЅљ÷÷“ї¬»»°іъ≤ъќпќ™
 (E)£ђ”…”Џ÷ї”–“ї¬»»°іъќпЋЃљв…ъ≥…“їќїіЉ£ђЈљњ…ЈҐ…ъЅђ–ш—хїѓ£ђі”ґш»Јґ®Bќ™
(E)£ђ”…”Џ÷ї”–“ї¬»»°іъќпЋЃљв…ъ≥…“їќїіЉ£ђЈљњ…ЈҐ…ъЅђ–ш—хїѓ£ђі”ґш»Јґ®Bќ™ £ђFќ™
£ђFќ™ £ђGќ™
£ђGќ™ £ђHќ™
£ђHќ™ .
. £ђ∆д÷–ЉЊC
£ђ∆д÷–ЉЊC …ѕ»эЄц°™CH3…ѕµƒ9ЄцH «µ»–Іµƒ£ђЉ”°∞°§°±µƒC…ѕµƒH≤їЌђ£ђЈҐ…ъ»°іъЇу…ъ≥…»э÷÷≤їЌђµƒ“ї¬»»°іъќп£ђґю «ѕы»•µƒЈљ љ£ђЉі°∞ѕы»•ѕаЅЏC‘≠„”…ѕµƒH°±.”√ѕ¬ЌЉ±н Њ£ЇB.
…ѕ»эЄц°™CH3…ѕµƒ9ЄцH «µ»–Іµƒ£ђЉ”°∞°§°±µƒC…ѕµƒH≤їЌђ£ђЈҐ…ъ»°іъЇу…ъ≥…»э÷÷≤їЌђµƒ“ї¬»»°іъќп£ђґю «ѕы»•µƒЈљ љ£ђЉі°∞ѕы»•ѕаЅЏC‘≠„”…ѕµƒH°±.”√ѕ¬ЌЉ±н Њ£ЇB. ЇЌD.
ЇЌD. ЈҐ…ъѕы»•Јі”¶µƒ≤ъќпЊщќ™
ЈҐ…ъѕы»•Јі”¶µƒ≤ъќпЊщќ™ £ї»э «Ѕђ–ш—хїѓµƒћхЉю∞µ Њ£ђЉі
£ї»э «Ѕђ–ш—хїѓµƒћхЉю∞µ Њ£ђЉі


 .
.

 £ђ
£ђ

