��Ŀ����
9�� Ϊ�ⶨij����þ�Ͻ𣨲�������Ԫ�أ���þ�������������о���ѧϰС�����λͬѧ������������ֲ�ͬʵ�鷽������̽������д���пհף�
Ϊ�ⶨij����þ�Ͻ𣨲�������Ԫ�أ���þ�������������о���ѧϰС�����λͬѧ������������ֲ�ͬʵ�鷽������̽������д���пհף���̽��һ��
ʵ�鷽������þ�Ͻ����ռ���Һ��Ӧ���ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2��
ʵ�鲽�裺
��1����ȡ5.4g��þ�Ͻ��ĩ��Ʒ��Ͷ��amL2.0mol/LNaOH��Һ�г�ַ�Ӧ����a��100��
��2�����ˡ�ϴ�ӡ�����������壮�ò�������δϴ�ӹ��壬���þ������������ƫ�ߣ��ƫ�ߡ���ƫ�͡�����
��̽������
ʵ�鷽������þ�Ͻ������ᷴӦ���ⶨ��������������ʵ��װ����ͼ1��
�������ۣ�
��3��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�ã��������ǣ�����Ҫ�����Ҫ������Ҫ������
��4��Ϊʹ�ⶨ��������ܾ�ȷ������ʱӦע��������ǣ�д�����㣩���ٴ���ȴ�������ٶ����������������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ�������밼Һ����ʹ����У�ƽ�Ӷ�����
��̽������
ʵ�鷽��������x g��þ�Ͻ��ĩ��������ͼ2��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������գ�
�������ۣ�
��5����ʵ���л���ⶨ�����������պ���������
��6����O2�����ÿ����������ǵ�������þ��Ӧ���ɵ���þ��
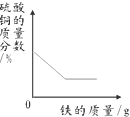
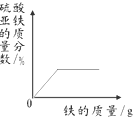
���� ̽��һ����������������Һ��Ӧ����ƫ��������������
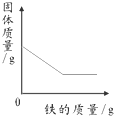
��1��þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
̽��������3���Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã�
��4������������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����������밼Һ����ʹ����У�ƽ�Ӷ����ȣ�
̽��������5��Mg��Al���ܹ���������ѧ��Ӧ���������
��6���ÿ�������O2����ʵ�飬������Ӧ��3Mg+N2$\frac{\underline{\;��ȼ\;}}{\;}$Mg3N2��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C���ⶨ���ɹ�����������
��� �⣺̽��һ����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��1����þΪ0ʱ���������ĺ�����ߣ�5.4g�Ͻ�����������Ϊ��5.4g����1-3%��=5.4��97%g����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 2mol
5.4g a��10-3L��2.0mol/L
����54g��5.4g=2mol����a��10-3L��2.0mol/L����
��ã�a=100mL��
��a��NaOH��Һ����100mL��
�ʴ�Ϊ��100��
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��þ����������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
̽��������3�������Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã��ʴ�Ϊ������Ҫ��
��4��Ϊʹ�ⶨ��������ܾ�ȷ������ʱӦע��������ǣ�����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ�����������밼Һ����ʹ����У�ƽ�Ӷ����ȣ��ʴ�Ϊ������ȴ�������ٶ����������������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ�������밼Һ����ʹ����У�ƽ�Ӷ����ȣ�
��̽��������
��5��Mg��Al����������Ӧ�����ɽ������������ⶨ���������������ʵ���л���ⶨ�������ǣ����պ������������ʴ�Ϊ�����պ�����������
��6�����ÿ�������O2����ʵ�飬������Ӧ��3Mg+N2 $\frac{\underline{\;��ȼ\;}}{\;}$Mg3N2��2Mg+CO2 $\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C����������þ��Ӧ���ɵ���þ���ⶨ���ɹ�������������þ����������ƫ�ߣ��ʴ�Ϊ����������þ��Ӧ���ɵ���þ��
���� ���⿼�����ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������

| A�� | 10mL 18mol/LŨ����������ͭ���ȳ�ַ�Ӧ��ת�Ƶ�����Ϊ0.18NA | |
| B�� | 0.1mol24Mg32S������������������Ϊ2.8NA | |
| C�� | ��״���£�22.4LCO2�к��еĹ�ͬ���Ӷ���Ϊ2NA | |
| D�� | 6.0g���ᾧ���к��е�H+��Ϊ0.1NA |
| A�� | 0.2mol | B�� | 0.5mol | C�� | 2mol | D�� | 5mol |
| A�� | ������������ķ�ˮ�����ŷ� | |
| B�� | ũҵ������ũҩ�����ʵĴ���ʹ�� | |
| C�� | ʵ��ú��������ã�������������Դ | |
| D�� | ʹ�ú��м�ȩ��뱵ȵIJ���װ���� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | CH2O��C2H4O2��C6H12O6 | B�� | CH2=CH2��C2H5OH��HOCH2CH2COOH | ||
| C�� | C6H6O��C5H10��C7H6O2 | D�� | H2��CO��CH3OH |
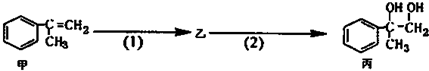
| A�� | ���п��ܺ���δ��Ӧ�ļף�������ˮ�����Ƿ� | |
| B�� | ��Ӧ��1�������Լ���Һ�壬�������� | |
| C�� | �ͱ�����������KMnO4��Һ������Ӧ | |
| D�� | ��Ӧ��2����Ӧ����ȡ����Ӧ |
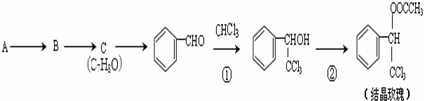
 ��
��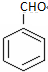 �ĺ˴Ź�������ͼ����4��壮
�ĺ˴Ź�������ͼ����4��壮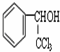 +CH3COOH$��_{��}^{Ũ����}$
+CH3COOH$��_{��}^{Ũ����}$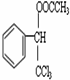 +H2O��
+H2O��