ÌâÄ¿ÄÚÈÝ
¡¾ÌâÄ¿¡¿
¢ñ£®Ëá¼îÖк͵ζ¨¡ª¡ªÒÑ֪ijNaOHÊÔÑùÖк¬ÓÐNaClÔÓÖÊ£¬Îª²â¶¨ÊÔÑùÖÐNaOHµÄÖÊÁ¿·ÖÊý£¬½øÐÐÈçϲ½ÖèʵÑ飺
¢Ù³ÆÁ¿1.00gÑùÆ·ÈÜÓÚË®£¬Åä³É250 mLÈÜÒº£»
¢Ú׼ȷÁ¿È¡25.00mLËùÅäÈÜÒºÓÚ׶ÐÎÆ¿ÖУ»
¢Û µÎ¼Ó¼¸µÎ·Ó̪ÈÜÒº£»
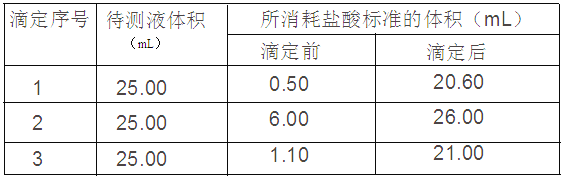
¢ÜÓÃ0.10mol£¯LµÄ±ê×¼ÑÎËáµÎ¶¨Èý´Î£¬Ã¿´ÎÏûºÄÑÎËáµÄÌå»ý¼Ç¼ÈçÏ£º
£¨1£©ÓÃ___________µÎ¶¨¹Ü£¨Ìî¡°Ëáʽ¡±»ò¡°¼îʽ¡±£©Ê¢×°0.10mol/LµÄÑÎËá±ê×¼Òº¡£
£¨2£©ÊÔÑùÖÐNaOHµÄÖÊÁ¿·ÖÊýΪ______________________¡£
£¨3£©Èô³öÏÖÏÂÁÐÇé¿ö£¬²â¶¨½á¹ûÆ«¸ßµÄÊÇ___________¡£
a£®µÎ¶¨Ç°ÓÃÕôÁóË®³åϴ׶ÐÎÆ¿
b£®ÔÚÕñµ´×¶ÐÎƿʱ²»É÷½«Æ¿ÄÚÈÜÒº½¦³ö
c£®ÈôÔڵζ¨¹ý³ÌÖв»É÷½«ÊýµÎËáÒºµÎÔÚ׶ÐÎÆ¿Íâ
d£®ËáʽµÎ¶¨¹ÜµÎÖÁÖÕµã¶Ô£¬¸©ÊÓ¶ÁÊý
e£®ËáʽµÎ¶¨¹ÜÓÃÕôÁóˮϴºó£¬Î´Óñê×¼ÒºÈóÏ´
¢ò£®Ñõ»¯»¹ÔµÎ¶¨¡ªÈ¡²ÝËáÈÜÒºÖÃÓÚ׶ÐÎÆ¿ÖУ¬¼ÓÈëÊÊÁ¿Ï¡ÁòËᣬÓÃŨ¶ÈΪ0.1mol¡¤L£1µÄ¸ßÃÌËá¼ØÈÜÒºµÎ¶¨£¬·¢ÉúµÄ·´Ó¦Îª£º2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2¡ü+2MnSO4+8H2O¡£
£¨4£©µÎ¶¨Ê±£¬KMnO4ÈÜҺӦװÔÚËáʽµÎ¶¨¹ÜÖУ¬µÎ¶¨ÖÕµãʱµÎ¶¨ÏÖÏóÊÇ________________¡£
¢ó£®³ÁµíµÎ¶¨¨D¨DµÎ¶¨¼ÁºÍ±»µÎ¶¨ÎïµÄÉú³ÉÎï±ÈµÎ¶¨¼ÁÓëָʾ¼ÁµÄÉú³ÉÎï¸üÄÑÈÜ¡£
£¨5£©²Î¿¼Ï±íÖеÄÊý¾Ý£¬ÈôÓÃAgNO3µÎ¶¨NaSCNÈÜÒº£¬¿ÉÑ¡ÓõÄָʾ¼ÁÊÇ £¨ÌîÑ¡Ïî×Öĸ£©¡£
ÄÑÈÜÎï | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
ÑÕÉ« | °× | dz»Æ | °× | שºì | °× |
Ksp | 1.77¡Á10£10 | 5.35¡Á10£13 | 1.21¡Á10£16 | 1.12¡Á10£12 | 1.0¡Á10£12 |
A£®NaCl B£®NaBr C£®NaCN D£®Na2CrO4
¡¾´ð°¸¡¿£¨1£©Ëáʽ £¨2£© 80% £¨3£© ce
£¨4£©×¶ÐÎÆ¿ÖÐÈÜÒºÓÉÎÞÉ«±äΪdzºìÉ«»òdz×ÏÉ«£¬ÇÒ°ë·ÖÖÓÄÚ²»ÍÊÉ« £¨5£©D
¡¾½âÎö¡¿ÊÔÌâ·ÖÎö£º£¨1£©ÔÚËá¼îÖк͵ζ¨ÖУ¬ÓÃËáʽµÎ¶¨¹ÜÊ¢×°ËáÐÔ»ò¾ßÓÐÑõ»¯ÐÔµÄÈÜÒº£¬ÓüîʽµÎ¶¨¹ÜÊ¢×°¼îÐÔÒºÌ壬ËùÒÔÓÃËáʽµÎ¶¨¹ÜÊ¢×°0.10mol/LµÄÑÎËá±ê×¼Òº£»
£¨2£©¸ù¾ÝÈý´ÎʵÑéËùµÃÊý¾ÝÇóƽ¾ùÖµ£¬ÏûºÄÑÎËáÌå»ýΪ£¨20.10+20.00+19.90£©¡Â3=20.00mL£¬ÔÙ¸ù¾Ýc£¨Ëᣩ¡ÁV£¨Ëᣩ=c£¨¼î£©¡ÁV£¨¼î£©£¬0.1¡Á20=c£¨¼î£©¡Á25£¬½âµÃc£¨¼î£©=0.08mol/L£¬ÊÔÑùÖÐNaOHµÄÖÊÁ¿·ÖÊýΪ[£¨0.08¡Á0.25£©¡Á40]¡Â1¡Á100%=80%£»
£¨3£©a£®µÎ¶¨Ç°ÓÃÕôÁóË®³åϴ׶ÐÎÆ¿£¬¶Ô²â¶¨½á¹ûÎÞÓ°Ï죻b£®ÔÚÕñµ´×¶ÐÎƿʱ²»É÷½«Æ¿ÄÚÈÜÒº½¦³ö£¬µ¼Ö¼îÒº¼õÉÙ£¬ÏûºÄ±ê×¼Òº¼õÉÙ£¬Ëù²âŨ¶ÈÆ«µÍ£»c£®ÈôÔڵζ¨¹ý³ÌÖв»É÷½«ÊýµÎËáÒºµÎÔÚ׶ÐÎÆ¿Í⣬µ¼ÖÂÏûºÄ±ê×¼ËáÒºÔö´ó£¬Ëù²âŨ¶ÈÆ«¸ß£»d£®ËáʽµÎ¶¨¹ÜµÎÖÁÖÕµã¶Ô£¬¸©ÊÓ¶ÁÊý£¬µ¼ÖÂÄ©¶ÁÊý¾Ý¼õС£¬Ëù²â½á¹ûÆ«µÍ£¬e£®ËáʽµÎ¶¨¹ÜÓÃÕôÁóˮϴºó£¬Î´Óñê×¼ÒºÈóÏ´£¬µ¼Ö±ê׼ҺŨ¶È½µµÍ£¬ÏûºÄ±ê×¼ÒºÌå»ýÔö´ó£¬Ëù²â½á¹ûÆ«¸ß£»´ð°¸Ñ¡ce£»
£¨4£©KMnO4ÈÜÒºÊÇ×ÏÉ«ÈÜÒº£¬µ±¸ßÃÌËá¼ØµÎÈ룬ÓÉÓÚ·´Ó¦µ¼ÖÂÈÜÒºÎÞÉ«£¬Èôµ½Öյ㣬ÔÙµÎÈëµÄ¸ßÃÌËá¼ØʹÈÜÒº³Ê×ÏÉ«£¬ËùÒԸõζ¨¹ý³Ì²»ÐèҪָʾ¼Á£¬µÎ¶¨ÖÕµãʱµÎ¶¨ÏÖÏóÊÇ׶ÐÎÆ¿ÖÐÈÜÒºÓÉÎÞÉ«±äΪdzºìÉ«»òdz×ÏÉ«£¬ÇÒ°ë·ÖÖÓÄÚ²»ÍÊÉ«£»
£¨5£©ÈôÓÃAgNO3µÎ¶¨NaSCNÈÜÒº,ÓÉÓÚ²úÉúµÄAgSCNµÄÈܶȻý³£ÊýÊÇ1.0¡Á10£12£¬¸ù¾ÝÈܶȻý³£Êý¿ÉÖª²úÉúAg2CrO4µÄÈܶȻý²úÉúÊÇ1.12¡Á10£12£¬ÐèÒªµÄCrO42-µÄŨ¶È´ó£¬»»ÑÔÖ®AgSCN±ÈAg2CrO4¸üÄÑÈÜ£¬¶øÆäËüÎïÖʱÈAgSCNÄÑÈÜ£¬Òò´ËÑ¡ÔñµÄָʾ¼ÁÊÇNa2CrO4£¬´ð°¸Ñ¡D¡£

¡¾ÌâÄ¿¡¿CO2ÊÇÄ¿Ç°×îÖ÷ÒªµÄÎÂÊÒÆøÌ壬¼õСCO2µÄÅŷŲ¢ÓÃÀ´ÖÆÔìÓмÛÖµµÄ»¯Ñ§ÓÃÆ·ÊÇÄ¿Ç°µÄÑо¿Ä¿±ê¡£
£¨1£©ÀûÓÃCO2ÓëCH4Éú²úºÏ³ÉÆø(CO¡¢H2)£º
ÒÑÖª£ºCH4(g)+2O2(g)![]() CO2(g)+2H2O(g) ¦¤H=£890.3 KJ¡¤mol£1
CO2(g)+2H2O(g) ¦¤H=£890.3 KJ¡¤mol£1
CO(g)+H2O(g)![]() CO2(g)+H2(g) ¦¤H=+2.8 KJ¡¤mol-1
CO2(g)+H2(g) ¦¤H=+2.8 KJ¡¤mol-1
2CO(g)+O2(g)![]() 2CO2(g) ¦¤H=£566.0 KJ¡¤mol£1
2CO2(g) ¦¤H=£566.0 KJ¡¤mol£1
·´Ó¦CO2(g)+CH4(g)![]() 2CO(g)+2H2(g) ¦¤H= ____________¡£
2CO(g)+2H2(g) ¦¤H= ____________¡£
¢Ú250¡æʱ£¬ÒÔÄøºÏ½ðΪ´ß»¯¼Á£¬ÏòÌå»ýΪ4 LµÄÃܱÕÈÝÆ÷ÖÐͨÈë6 mol CO2¡¢6 mol CH4£¬¿ªÊ¼·¢ÉúÈçÏ·´Ó¦£ºCO2(g)+CH4(g)![]() 2CO(g)+2H2(g)¡£¾¹ýÒ»¶Îʱ¼ä´ïµ½Æ½ºâ£¬Æ½ºâÌåϵÖи÷×é·ÖÌå»ý·ÖÊý(ijһ³É·ÖÎïÖʵÄÁ¿Õ¼×ÜÆøÌåÎïÖʵÄÁ¿µÄ°Ù·ÖÊý)ÈçÏÂ±í£º
2CO(g)+2H2(g)¡£¾¹ýÒ»¶Îʱ¼ä´ïµ½Æ½ºâ£¬Æ½ºâÌåϵÖи÷×é·ÖÌå»ý·ÖÊý(ijһ³É·ÖÎïÖʵÄÁ¿Õ¼×ÜÆøÌåÎïÖʵÄÁ¿µÄ°Ù·ÖÊý)ÈçÏÂ±í£º
ÎïÖÊ | CH4 | CO2 | CO | H2 |
Ìå»ý·ÖÊý | 0.1 | 0.1 | 0.4 | 0.4 |
´ËζÈϸ÷´Ó¦µÄƽºâ³£ÊýK=________________¡£
£¨2£©ÒÔ¶þÑõ»¯îѱíÃ渲¸ÇCu2Al2O4Ϊ´ß»¯¼Á£¬¿ÉÒÔ½«CO2ºÍCH4Ö±½Óת»¯³ÉÒÒËá¡£ÔÚ²»Í¬Î¶ÈÏ´߻¯¼ÁµÄ´ß»¯Ð§ÂÊÓëÒÒËáµÄÉú³ÉËÙÂÊÈçÏÂͼËùʾ¡£250¡«300¡æʱ£¬ÒÒËáµÄÉú³ÉËÙÂʼõСµÄ¿ÉÄÜÔÒòÊÇ____________________¡£

£¨3£© ÈçÒÔÇâÑõ»¯¼ØË®ÈÜÒº×÷µç½âÖʽøÐеç½â£¬CO2ÔÚ͵缫ÉÏ¿Éת»¯Îª¼×Í飬¸Ãµç¼«·´Ó¦·½³ÌʽΪ_____________________¡£
£¨4£©½«2mol CO2ºÍ6molH2ÈÝ»ýÏàͬ¶øζȲ»Í¬µÄ¢ñ¡¢¢òÁ½¸öºãÈÝÃܱÕÈÝÆ÷ÖпªÊ¼·¢Éú·´Ó¦£ºCO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g)£¬²âµÃCH3OHµÄÎïÖʵÄÁ¿Ëæʱ¼äµÄ±ä»¯ÈçÏÂͼ1Ëùʾ¡£
CH3OH(g)+H2O(g)£¬²âµÃCH3OHµÄÎïÖʵÄÁ¿Ëæʱ¼äµÄ±ä»¯ÈçÏÂͼ1Ëùʾ¡£


¢ÙÇúÏߢñ¡¢¢ò¶ÔÓ¦µÄƽºâ³£Êý´óС¹ØϵΪK¢ñ_______K¢ò(Ìî¡°>¡±¡°=¡±»ò¡°<¡±)£¬¿ÉÖª¸Ã ·´Ó¦ÊÇÒ»¸ö_______(Ìî¡°·ÅÈÈ¡±»ò¡°ÎüÈÈ¡±)·´Ó¦¡£
¢ÚÏÂÁÐÊÂʵ˵Ã÷¸Ã·´Ó¦ÒѴﵽƽºâ״̬µÄÊÇ_________________£º
A£®ÈÝÆ÷ÄÚÆøÌåѹǿ±£³Ö²»±ä
B£®ÈÝÆ÷ÄÚÆøÌåµÄÃܶȱ£³Ö²»±ä
C£®CO2µÄÌå»ý·ÖÊý±£³Ö²»±ä
D£®CO2µÄÏûºÄËÙÂÊÓëCH3OHµÄÉú³ÉËÙÂÊÏàµÈ
E£®ÈÝÆ÷ÄÚ»ìºÏÆøÌåµÄƽ¾ùÏà¶Ô·Ö×ÓÖÊÁ¿±£³Ö²»±ä
£¨5£©ÀûÓùâÄܺ͹â´ß»¯¼Á£¬¿É½«CO2ºÍH2O(g)ת»¯ÎªCH4ºÍO2¡£×ÏÍâ¹âÕÕÉäʱ£¬ÔÚ²»Í¬´ß»¯¼Á(¢ñ¡¢¢ò¡¢¢ó)×÷ÓÃÏ£¬CH4²úÁ¿Ëæ¹âÕÕʱ¼äµÄ±ä»¯ÈçÉÏͼ2Ëùʾ¡£ÔÚ0¡«15СʱÄÚ£¬CH4µÄƽ¾ùÉú³ÉËÙÂÊ¢ñ¡¢¢òºÍ¢ó´Ó´óµ½Ð¡µÄ˳ÐòΪ_____________(ÌîÐòºÅ)¡£