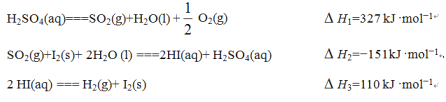��Ŀ����
����Ŀ��±�����������ܼ�����Mg��Ӧ���Ƶø����Լ��������Լ����л��ϳɷ�����;�㷺����RΪ��������֪��![]()

��ij�л���A������ת����ϵ��

�Իش��������⣺
��1��B��A�ķ�Ӧ������______________________________
��2��C��D�Ļ�ѧ��Ӧ����ʽ�� _____________________
��3��G�Ľṹ��ʽ��_______________________________
��4��I�����������ŵ�������__________________
��5������ʽ��I��J��ͬ�����ܷ���������Ӧ���칹����_________�֡�д�����к�-CH3���������칹��Ľṹ��ʽ_______________________________________
��6���۱���ϩ��PS����һ�ֶ�����ϣ��㷺Ӧ����ʳƷ��װ����Ե�壬��ҵ�����豸�������ճ����������С�д���� D�ͱ�Ϊ��Ҫԭ���Ʊ��۱���ϩ�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
 ____________
____________
���𰸡� ��ȥ��Ӧ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O H3CCH(OH)CH2CH2CH(OH)CH3 ̼̼˫�����ǻ� 8 (CH3)3CCH2CHO
2CH3CHO+2H2O H3CCH(OH)CH2CH2CH(OH)CH3 ̼̼˫�����ǻ� 8 (CH3)3CCH2CHO 
��������������������⿼���л��ƶϣ��л���ṹ��ʽ���л�����ʽ����д��ͬ���칹�����д�������ȷ�����л��ϳ�·�ߵ���ơ�A��Br2/CCl4��Ӧ����E��E�ķ���ʽΪC2H4Br2����A�Ľṹ��ʽΪCH2=CH2��E�Ľṹ��ʽΪBrCH2CH2Br��E��F���������Ϣ�ķ�Ӧ��FΪBrMgCH2CH2MgBr��FΪ�����Լ���������Ϣ�����Լ��뺬![]() �����ʷ����ӳɷ�Ӧ��F+D��G��D�к�
�����ʷ����ӳɷ�Ӧ��F+D��G��D�к�![]() �����G�ķ���ʽC6H14O2���Ƴ�D�Ľṹ��ʽΪCH3CHO��G�Ľṹ��ʽΪ
�����G�ķ���ʽC6H14O2���Ƴ�D�Ľṹ��ʽΪCH3CHO��G�Ľṹ��ʽΪ![]() ������B��A��CH2=CH2����B��C��D��CH3CHO���������л���֮����ת�����Ƴ�BΪCH3CH2Br����CH3CH2Cl����CΪCH3CH2OH��
������B��A��CH2=CH2����B��C��D��CH3CHO���������л���֮����ת�����Ƴ�BΪCH3CH2Br����CH3CH2Cl����CΪCH3CH2OH��
��1��B��A��CH3CH2Br����CH3CH2Cl����CH2=CH2Ϊ±������NaOH����Һ�е���ȥ��Ӧ��
��2��C��D�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��3��G�Ľṹ��ʽΪ![]() ��
��
��4��G���ڱ��Ͷ�Ԫ����I��J��Ϊͬ���칹�壬�Ա�I��J��G�ķ���ʽ��I��J����G��Ũ���ᡢ������������ȥһ����ˮ������ J����Ԫ��״�����J�Ľṹ��ʽΪ![]() ����IΪG��ȥһ����ˮ�õ���I�еĹ����ŵ�����Ϊ�ǻ���̼̼˫����
����IΪG��ȥһ����ˮ�õ���I�еĹ����ŵ�����Ϊ�ǻ���̼̼˫����
��5������ʽ��I��J��ͬ�����ܷ���������Ӧ���칹���д��C5H11CHO����ͬ���칹��Ľṹ��ʽΪCH3CH2CH2CH2CH2CHO����CH3��2CHCH2CH2CHO��CH3CH2CH��CH3��CH2CHO��CH3CH2CH2CH��CH3��CHO����CH3CH2��2CHCHO����CH3��3CCH2CHO��CH3CH2C��CH3��2CHO����CH3��2CHCH��CH3��CHO������8�֡����к�-CH3���������칹��Ľṹ��ʽΪ��CH3��3CCH2CHO��CH3CH2C��CH3��2CHO����CH3��2CHCH��CH3��CHO��
��6������������������ϳɾ۱���ϩ��ϳ��䵥�屽��ϩ���Աȱ���ϩ�ͱ���D��CH3CHO����̼����Ҫ������CH3CHO��̼������������D�к�![]() ��������Ϣ��Ӧ���ɱ��ϳ��屽���屽��Mg/�������úϳɸ����Լ�
��������Ϣ��Ӧ���ɱ��ϳ��屽���屽��Mg/�������úϳɸ����Լ�![]() �������Լ�
�������Լ�![]() ��CH3CHO������Ϣ�з�Ӧ����
��CH3CHO������Ϣ�з�Ӧ����![]() ��
��![]() ������ȥ��Ӧ�ñ���ϩ���ϳ�·������ͼΪ��
������ȥ��Ӧ�ñ���ϩ���ϳ�·������ͼΪ��  ��
��

����Ŀ��Ϊ�ᴿ�������ʣ�������Ϊ���ʣ������������ˮ���������йس����Լ��ͷ��뷽����ѡ����ȷ���ǣ� ��
ѡ�� | ���ᴿ������ | �����Լ� | ���뷽�� |
A | CH3CH3��CH2=CH2�� | ��ˮ | ϴ�� |
B | CH3CH2Br��Br2�� | NaOH��Һ | ��Һ |
C | �屽������ | ���� | |
D | ��ϩ(�Ҵ�����) | ˮ | ϴ�� |
A.AB.BC.CD.D