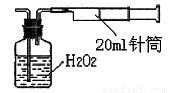
��Ŀ����
��12�֣�����ͼ�ס����ǵ绯ѧʵ��װ�á�
(1) ���ס������ձ��о�ʢ��NaCl��Һ��
�� ����ʯī���ϵĵ缫��ӦʽΪ____________________________________________��
�� ��ʪ��ĵ���KI��ֽ�������ձ��Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
(2) ���ס������ձ��о�ʢ��CuSO4��Һ��
�� ���������ϵĵ缫��ӦʽΪ______________________________________________��
�������ʼʱ����ʢ��200 mL pH��5��CuSO4��Һ(25 ��)��һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ������ǰ��״̬��������Һ�м���_____ (��д���ʵĻ�ѧʽ)________g��
(12��)��(1)��O2��2H2O��4e��===4OH����2�֣���5Cl2��I2��6H2O===10HCl��2HIO3��3�֣�
(2)��Fe��2e��===Fe2�� ��2�֣���C CuO(��CuCO3) ��2�֣� ��0.8(��1.24) ��3�֣�
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
��Ӧ��ϵ�и���ֵIJ������ʼ��±���
���� | ���� | ���� | �� |
�۵�/�� | -210.01 | -252.77 | -77.74 |
�е�/�� | -195.79 | -259.23 | -33.42 |
(1)�ϳɰ���Ӧ��ƽ�ⳣ����С�������ڹ�ҵ�ϲ�ȡ����ѭ�������̣�����Ӧ��ͨ���ѻ��������¶Ƚ��͵�____________��ʹ�������������Ʒ��N2��H2����ѭ����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=____________��
(2)���û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ҵ�ϲ��á�ʹ�������������Ʒ��N2��H2����ѭ������������ʩ�����ɣ�___________________________________________________��
(3)��֪��1 mol H��H����1 mol N��H����1 mol N��N���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ_____________________________��
(4)�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣�����ܱ������еķ�Ӧ��N2(g)+3H2(g) ![]() 2NH3(g)����673 K��30 MPa�£�n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ����ʾ������������ȷ����____________��
2NH3(g)����673 K��30 MPa�£�n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ����ʾ������������ȷ����____________��
A.��a������Ӧ���ʱȵ�b�Ĵ�
B.��c����Ӧ�ﵽƽ��
C.��d(t1ʱ��)�͵�e(t2ʱ��)��n(N2)��һ��
D.�����������䣬773 K�·�Ӧ��t1ʱ�̣�n(H2)��ͼ��d���ֵ��
(5)1998��ϣ������˹��´�ѧ��Marncellos��Stoukides���ø����ӵ����Ե�SCY�մ�(�ܴ���H+)��ʵ���˸��³�ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����ʵ��װ������ͼ�ҡ������ĵ缫��ӦʽΪ__________________________________________________________________��


�� ��