��Ŀ����
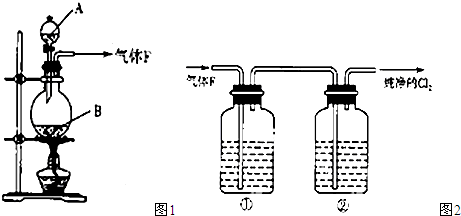
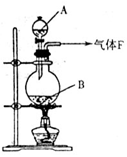
��12�֣� ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⣺
��1��д��A�з�����Ӧ�Ļ�ѧ����ʽ ��
��2���� װ��B��ʢ�ŵ��Լ�Ϊ �������� ��
��װ��D��E�г��ֵIJ�ͬ����˵���������� ��
��д��װ��G�з�����Ӧ�����ӷ���ʽ ��
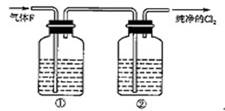
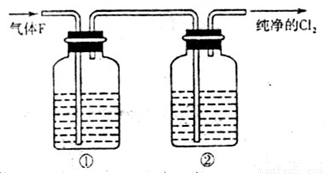
��3����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�һ��װ�á�����Ϊ��װ��Ӧ���� �� ֮�䣨������װ����ĸ��ţ���װ����Ӧ����
����д�Լ�����Ʒ���ƣ���
��4����ͬѧ��4.48L��������״���²ⶨ��ͨ�뵽������ʯ�����У������Ͽ��Ƶ������������� g��
��1��4HCl(Ũ)ʮMn02  MnCl2+C12��+2H2O��
MnCl2+C12��+2H2O��
��2���� ��ˮ����ͭ��֤����ˮ��������������������Ҳ���֣��� ��������Ư���ԣ���������Ư���ԣ������������� ��Ag++Cl��=AgCl����
��3��F G�� ʪ��ĵ���KI��ֽ����ʪ�����ɫ������ �� �� 14.3
������������������Ŀ����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ�����д��A�з�����Ӧ�Ļ�ѧ����ʽ��4HCl(Ũ)ʮMn02  MnCl2+C12��+2H2O���� װ��B��ʢ�ŵ��Լ�Ϊ��ˮ����ͭ��������֤����ˮ�������� ��
MnCl2+C12��+2H2O���� װ��B��ʢ�ŵ��Լ�Ϊ��ˮ����ͭ��������֤����ˮ�������� ��
��װ��D��E�г��ֵIJ�ͬ����˵���������Ǣ�������Ư���ԣ���������Ư���ԣ�������������
��д��װ��G�з�����Ӧ�����ӷ���ʽAg++Cl��=AgCl����
��3����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����ȷ������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�һ��װ�á�����Ϊ��װ��Ӧ����F�� G֮�䣬��ȥ���ܵ�������װ����Ӧ����ʪ��ĵ���KI��ֽ��ʪ�����ɫ�����������Dz����������Ĵ���;��4����ͬѧ��4.48L��������״���²ⶨ��ͨ�뵽������ʯ�����У������Ͽ��Ƶ�������������14.3g������������£�
2 Cl2 + 2 Ca(OH)2 = CaCl2 + Ca(ClO)2 +2 H2O
2 ��22.4 143
4.48 x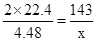 ����x=14.3
����x=14.3
���㣺���������ʺ�ʵ��
����������ʵ�飬һ��Ҫȷ����ʵ���Ŀ�ģ�ʵ���ÿһ�������Ͳ��趼��Ϊ�˱�֤ʵ��Ŀ�ĵĴ�ɡ�

 ���ӷ���ʽΪ______________________________________________��
���ӷ���ʽΪ______________________________________________��
 ���ʵ���Ϊ____________________��
���ʵ���Ϊ____________________��