̀âÄ¿ÄÚÈƯ
ijÎ̃É«ÈÜ̉º£¬ÓÉNa£«¡¢Ag£«¡¢Ba2£«¡¢Al3£«¡¢AlO2¡ª¡¢MnO4¡ª¡¢CO32¡ª¡¢SO42¡ª¡¢SiO32¡ªÖеÄÈô
¸ÉÖÖ×é³É(²»¿¼ÂÇË®µÄµçÀë)¡£È¡¸ĂÈÜ̉º½øĐĐÈçÏÂʵÑ飺
¢ñ.È¡ÊÊÁ¿ÈÜ̉º£¬¼ÓÈë¹ưÁ¿µÄÑÎËᣬÓĐÆø̀åÉú³É£¬²¢µĂµ½Î̃É«ÈÜ̉º£»
¢̣.ÔÚ¢ñËùµĂÈÜ̉ºÖĐ¼ÓÈë¹ưÁ¿µÄNH4HCO3ÈÜ̉º£¬ÓĐÆø̀åÉú³É£¬Í¬Ê±Îö³ö°×É«³Áµí¼×£»
¢ó.ÔÚ¢̣ËùµĂÈÜ̉ºÖĐ¼ÓÈë¹ưÁ¿µÄBa(OH)2ÈÜ̉º£¬¼ÓÈÈ£¬̉²ÓĐÆø̀åÉú³É£¬Í¬Ê±Îö³ö°×É«³Áµí̉̉¡£
Çë»Ø´đÏÂÁĐÎỀ⣺
(1)ÓÉʵÑé¢ñ¿ÉÖªÔÈÜ̉ºÖĐ̉»¶¨²»º¬ÓеÄÀë×ÓÊÇ________£»̉»¶¨º¬ÓеÄÀë×ÓÊÇ________________¡£
(2)ÓÉʵÑé¢̣¿ÉÖªÔÈÜ̉ºÖĐ»¹̉»¶¨º¬ÓеÄÀë×ÓÊÇ________£»Éú³É¼×µÄÀë×Ó·½³̀ʽΪ________________¡£
(3)ʵÑé¢óÖĐÉú³É°×É«³Áµí̉̉µÄÀë×Ó·½³̀ʽΪ________________¡£
(4)ÔÈÜ̉ºÖĐ»¹¿ÉÄÜ´æÔÚµÄÀë×ÓÊÇ________£»¼́Ñé¸ĂÀë×ӵķ½·¨ÊÇ_________________¡£
(1)MnO4¡ª¡¢SiO32¡ª¡¢Ag£«¡¢Ba2£«¡¢Al3£«¡¡CO32¡ª¡¢Na£«
(2)AlO2¡ª¡¡3HCO3¡ª£«Al3£«=Al(OH)3¡ư£«3CO2¡ü
(3)2OH££«NH4+£«HCO3¡ª£«Ba2£«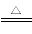 BaCO3¡ư£«NH3¡ü£«2H2O
BaCO3¡ư£«NH3¡ü£«2H2O
(4)SO42¡ª¡¡È¡°×É«³Áµí̉̉ÉÙĐí£¬·ÅÈëÊÔ¹ÜÖĐ£¬¼ÓÈë¹ưÁ¿µÄÏ¡ÑÎËá»̣Ï¡ÏơËᣬÈôÈÔÓĐ²¿·Ö°×É«³Áµí²»Èܽ⣬ỘÖ¤Ă÷ÔÈÜ̉ºÖĐÓĐSO42¡ª£»Èô°×É«³ÁµíÈ«²¿Èܽ⣬ỘÖ¤Ă÷ÔÈÜ̉ºÖĐÎ̃SO42¡ª
¡¾½âÎö¡¿¼ÓÈë¹ưÁ¿µÄÑÎËᣬÓĐÆø̀åÉú³É£¬²¢µĂµ½Î̃É«ÈÜ̉º£¬¿ÉÖªÈÜ̉ºÖĐ̉»¶¨²»º¬ÓĐÀë×ÓMnO4¡ª¡¢SiO32¡ª£¬̉»¶¨º¬ÓĐÀë×ÓCO32¡ª¡£ÓĐCO32¡ª£¬¾Í̉»¶¨²»º¬Ag£«¡¢Ba2£«¡¢Al3£«£¬ÓÉÓÚÈÜ̉º³ÊµçÖĐĐÔ£¬¿ÉÖªÈÜ̉ºÖĐ̉»¶¨º¬ÓĐÀë×ÓNa£«¡£ÔÚ¢ñËùµĂÈÜ̉ºÖĐ¼ÓÈë¹ưÁ¿µÄNH4HCO3ÈÜ̉º£¬ÓĐÆø̀åÉú³É£¬Í¬Ê±Îö³ö°×É«³ÁµíAl(OH)3£¬ËüÊÇAlO2¡ªÓë¹ưÁ¿µÄÑÎËá·´Ó¦Éú³ÉµÄAl3£«ÓëHCO3¡ªË«Ë®½âµĂµ½µÄ£¬¹ÊÔÈÜ̉ºÖĐ̉»¶¨»¹º¬ÓĐAlO2¡ª¡£