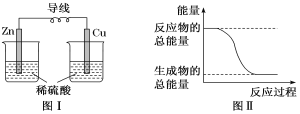
��Ŀ����
����Ŀ����A��B��C��D��E��F����Ԫ�أ���ԭ�ӵĺ˵������С��18�������ε�����Aԭ�Ӻ��ڽ���һ�����ӣ�Bԭ�ӵĵ���������Dԭ�ӵ�������������ȣ�Aԭ����Bԭ�ӵ�����������֮����Cԭ�ӵ�������������ȣ�D�������������Ǵ�����������3����E������������ˮ����������ԣ�F�ĵ�������;��㷺�İ뵼����ϡ�
���ƶ�����Ԫ�ز��ش��������⣺
(1)д����Ӧ��Ԫ�ط��ţ� B________��C________��D_______�� F________��
(2)B��C��D����̬�⻯����ȶ����ɴ�С��˳��Ϊ__________(�û�ѧʽ��ʾ)��
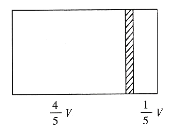
(3)����D�����ӽṹʾ��ͼ________��
(4)��һ����ѧ����ʽ֤��B��F�ķǽ����Ե�ǿ��____________________________��
(5)д��E������������ˮ������NaOH��Һ��Ӧ�����ӷ���ʽ��__________________��
(6)��Ԫ��D�γɵ�10���Ӽ����ӵķ���Ϊ________����Ԫ��F�γɵ�18���ӷ��ӵĻ�ѧʽΪ________��
���𰸡�C N O Si H2O>NH3>CH4 ![]() Na2SiO3+H2O+CO2=H2SiO3��+Na2CO3 Al(OH)3+OH- =AlO2-+2H2O O2- SiH4
Na2SiO3+H2O+CO2=H2SiO3��+Na2CO3 Al(OH)3+OH- =AlO2-+2H2O O2- SiH4
��������
��A��B��C��D��E��F����Ԫ�أ�����ԭ�ӵĺ˵������С��18�������ε�����Aԭ�Ӻ��ڽ���1�����ӣ���AΪHԪ�أ�Dԭ�ӵ������������Ǵ�����������3��������������������8��ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����DΪOԪ�أ�Bԭ�ӵĵ���������Dԭ�ӵ�������������ȣ���BΪCԪ�أ�Aԭ����Bԭ�ӵ�����������֮����Cԭ�ӵ�������������ȣ���Cԭ������������=1+4=5����ԭ������С��O����CΪNԪ�أ�E������������ˮ����������ԣ���EΪAlԪ�أ�F�ĵ�������;��㷺�İ뵼����ϣ���FΪSiԪ�ء�
�ɷ���֪��AΪHԪ�ء�BΪCԪ�ء�CΪNԪ�ء�DΪOԪ�ء�EΪAlԪ�ء�FΪSiԪ�أ�
(1)��Ԫ�ص�Ԫ��Ϊ�� BΪC��CΪN��DΪO�� FΪSi��
(2)C��N��O����Ԫ�صķǽ�����������ǿ������̬�⻯����ȶ����ɴ�С��˳��ΪH2O>NH3>CH4��
(3)O2-�����ӽṹʾ��ͼΪ![]() ��
��
(4) ��Na2SiO3+H2O+CO2=H2SiO3��+Na2CO3��֪̼������Աȹ���ǿ����C�ķǽ����Ա�Siǿ��
(5)Al(OH)3��NaOH��Һ��Ӧ�����ӷ���ʽΪAl(OH)3+OH- =AlO2-+2H2O��
(6)��Ԫ��O�γɵ�10���Ӽ����ӵķ���ΪO2-����Ԫ��Si�γɵ�18���ӷ��ӵĻ�ѧʽΪSiH4��