��Ŀ����
����Ŀ����ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�

(1)ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ490mL 4.6mol��L-1��ϡ���ᣬ��Ҫ���Ƹ�Ũ�ȵ���Һ����IJ�����������Ͳ���ձ�������������ͷ�ι��⣬����Ҫ__________(����������)����Ҫ��ȡ98%Ũ����____________mL�������ƣ�
(2)������Һʱ�����²�����a.ϡ���ܽ�b.ҡ��c.ϴ��d.��ȴe.��ȡf.����Һ��������ƿg.���ݣ�ʵ�����˳����ȷ������___________����
A. e��a��f��d��c��f��g��b B. e��a��d��f��c��f��g��b
C. e��a��f��d��c��f��b��g D. e��a��d��f��c��f��b��g
(3)����Ϊ���ƹ����в��ֲ�����ʾ��ͼ�������д������____(�����)��
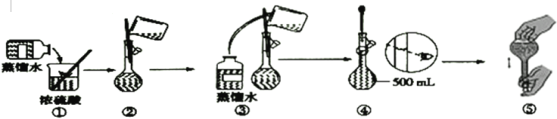
(4)������4.6mol��L-1ϡ����Ĺ����У���������������������õ�������Һ���ʵ���Ũ��ƫ�ߵ���___��
A.δ����ȴ���Ƚ���Һע������ƿ�� B.����ƿϴ�Ӻ�δ���ﴦ��
C.����ʱ���ӹ۲�Һ�� D.δϴ���ձ��Ͳ�����
(5)Ϊ�к�100mL 2.3 mol��L-1KOH��Һ�������ԣ���Ҫ����________mL 4.6mol��L-1ϡ���ᡣ
���𰸡�500mL����ƿ 125 B �٢� A 25
��������
(1)����c=![]() ����Ũ��������ʵ���Ũ�ȣ��ٸ���������Һ��һ�㲽���ж���Ҫ�������ͼ�����ҪŨ����������
����Ũ��������ʵ���Ũ�ȣ��ٸ���������Һ��һ�㲽���ж���Ҫ�������ͼ�����ҪŨ����������
(2)��������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ը�ѡ���������
(3)����Ũ����ϡ�͡����ݵ���ȷ���������жϣ�
(4)����c=![]() ������������
������������
(5)����2KOH��H2SO4���㡣
(1)��Ҫ490mL 4.6mol/L��ϡ���ᣬӦѡ��500mL����ƿ����Ũ��������һ�����ʵ���Ũ��ϡ���ᣬ��������Ϊ�����㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȣ��õ�����������Ͳ����ͷ�ιܡ��ձ�����������500mL����ƿ����ȱ�ٵ�������500mL����ƿ����������Ϊ98%���ܶ�Ϊ1.84g/mL��Ũ���ᣬ���ʵ���Ũ��c=![]() =
=![]() mol/L=18.4mol/L��ʵ������500mL��Һ������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ��������V��18.4mol/L=500mL��4.6mol/L�����V=125.0mL���ʴ�Ϊ��500mL����ƿ��125.0��
mol/L=18.4mol/L��ʵ������500mL��Һ������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ��������V��18.4mol/L=500mL��4.6mol/L�����V=125.0mL���ʴ�Ϊ��500mL����ƿ��125.0��
(2)����һ�����ʵ���Ũ����Һ�IJ����У����㡢��������ȡ���ܽ��ϡ�͡���ȴ��ת�ơ�ϴ��(ת��)�����ݡ�ҡ�ȵȣ�����ȷ�IJ���˳���ǣ�eadfcfgb���ʴ�Ϊ��B��
(3)ϡ��Ũ����ʱӦ�ý�Ũ���������ڵ���ˮ�У����ò��������Ͻ��裬ʹ����������Ѹ����ɢ������ʱ����Ӧƽ�ӣ��������Ӻ��ӣ��ʴ�Ϊ���٢ܣ�
(4)A��δ����ȴ���Ƚ���Һע������ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���Aѡ��B������ƿϴ�Ӻ�δ�����ﴦ���������ʵ����ʵ�������Һ�������������Ӱ�죬��ҺŨ�Ȳ��䣬��B��ѡ��C������ʱ���ӹ۲�Һ�棬������Һ���ƫ����ҺŨ��ƫ�ͣ���C��ѡ��D��δϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����ҺŨ��ƫС����D��ѡ���ʴ�Ϊ��A��
(5)����2KOH��H2SO4���к�100mL 2.3 mol/LKOH��Һ�������ԣ���Ҫ4.6mol/Lϡ��������Ϊ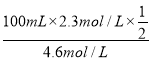 =25 mL���ʴ�Ϊ��25��
=25 mL���ʴ�Ϊ��25��