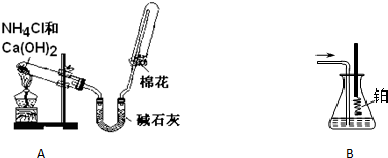
��Ŀ����
ijͬѧ������ʵ��װ�ü���ѧҩƷ��ȡ�������о������Ĵ����������㰴Ҫ��ش��������⣺
������������ ����������������
����������������
��������������������A ���� �������� B
��1����ͬѧ��ȡ�����Ļ�ѧ����ʽΪ ��
��2�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ�_________________��
��3��һ��ʱ����ռ��������Թܵ�����ˮ���У��۲쵽�Թ���Һ������ֻ�������Թܵ�һ�롣���������Թ���������ɢ��ˮ���У����״���£��Թ�����Һ�����ʵ���Ũ��Ϊ ��
��4����Bװ������һ��ʱ�䰱����ͨ�������ͬʱ�������ȵIJ�˿����Bװ�õ���ƿ�ڣ�д����ƿ�ڰ������Ļ�ѧ����ʽ ��
��5����Ӧ��������ƿ�ڵ���Һ�к���H����OH���� �� ���ӡ�
��1��2NH4Cl+Ca��OH��2===CaCl2+2NH3 ��+2H2O
��2����ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ռ���������պ��Ũ����IJ����������Թܿڣ����а��̲�������֤�����ռ�����
��3��1/22��4 ��0��045mol/L
��4��4NH3ʮ5O2![]() 4NO��6H2O ��5��NH4+ NO3-
4NO��6H2O ��5��NH4+ NO3-