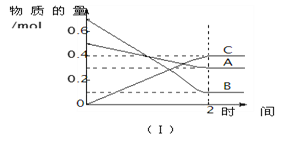
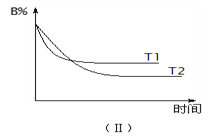
��Ŀ����
����Ŀ�������ֳ�ૼ��ᣬ��ʳƷ��ҵ�����������������ںϳ���֬��ҽҩ�����ϵȵ��� ���塣���ט�������о��ȡ��ૼ�ȩΪԭ�Ϻϳɿ��ᷴӦ����
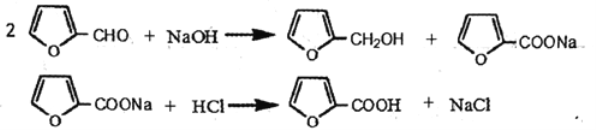
���������������:
���� | ��״ | ��Է� ������ | �ܽ�� | |||
��ˮ | ��ˮ | �Ҵ� | ���� | |||
ૼ�ȩ | ��ɫҺ�� | 96 | �� | ���� | ���� | ���� |
ૼ״� | ��ɫҺ�� | 98 | ���� | ���� | ���� | ���� |
ૼ��� | ��ɫ���� | 112 | �� | ���� | ���� | ���� |
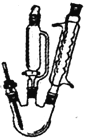
�չ�����ֽpH��ɫ��Χ��3.0(����ɫ)~5.0(��ɫ)
ૼ����ʵ�����Ʊ��������£�
����ƿ�з���3.28mL(0.04mol)����ૼ�ȩ������8~12���µμ�4mL40%NaOH��Һ����
�����������30���ӡ���Ӧ�����μ�����ˮ��Լ15mL)ʹ��ǡ�ó��壬��50mL������ȡ��Һ����ˮ��Һ��ˮ����������ȥ�ֳ�������μ�2:1���ᵽpH=3�����裬�����ᾧ�����˲�������ˮϴ�ӳ�ɣ��þ���Ʒ1.75g��
�ش��������⣺
(1) ����ƿ�з�Ӧ��Ҫ�������30���ӵ�ԭ����__________��
(2) ૼ�ȩ�ڼ��з�Ӧ�������ȣ�ʵ������Ҫ����8-12�棬���������_________��
(3)50mL������ȡ��Һ����ȥૼ״��IJ���������____________��
A�� ֱ����50mL������ȡ��Һ
B�� ����Һ�����ݣ�����Ҳ�����ݣ��ֱ���ȡ����ȡҺ�ϲ�
C�� ����30mL������ȡ��Һ���ٷֱ���l0mL������ȡ���Σ�����������ȡҺ�ϲ�
(4)������ȡ���ˮ��ҺҪ�������ữ������100mL 2��1ϡ����(ˮ�����������)�ķ�����___________���ж�������������ķ�����________________��
(5)�����ӷ�����ȼ��������ʹ��ʧȥ֪�����������������ʱע�⣺_____________��ˮ��������ķ���Ӧѡ��________(����ĸ���)��
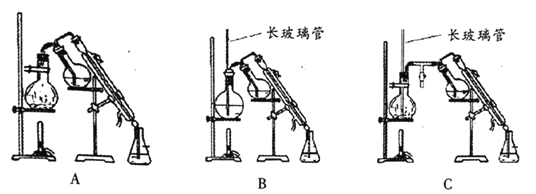
(6)��ૼ���IJ���___________________��
���𰸡� ૼ�ȩ����ˮ�������ֻ�ϼӿ췴Ӧ���� �����������𰸾����֣� ��ˮԡ ����ˮԡ�����������𰸾����֣� C ����Ͳ�ֱ���ȡ66mlˮ��34mlŨ���ᣬ���ձ��л�ϣ��ò������������ �����������𰸾����֣� ʹ�øչ�����ֽ�ⶨ��ҺpH������ֽ������ɫʱ��˵�������������� ���ϲ���Һ�м����μ����ᣬ���������ǣ�˵������������������������𰸾����֣� ��ˮԡ�Ͻ��������м�ֱ��������ȡ������������𰸾����֣� C 78.1%
��������(1) ૼ�ȩ����ˮ��Ϊ�˷�Ӧ������ֻ�ϲ��ӿ췴Ӧ���ʿɽ������һ��ʱ�䣻
(2) ૼ�ȩ�ڼ��з�Ӧ�������ȣ����Խ�����ˮԡ�������¶�8-12�����ң�
(3)��ȡ������ö�ν��У�����30mL������ȡ��Һ���ٷֱ���l0mL������ȡ���Σ�����������ȡҺ�ϲ��������ʴ�ΪC��
(4)����Ͳ�ֱ���ȡ66mlˮ��34mlŨ���ᣬ���ձ��л�ϣ��ò�����������ȼ��ɵõ�100mL 2��1ϡ������ϲ���Һ�м����μ����ᣬ���������ǣ�˵���������������ʹ�øչ�����ֽ�ⶨ��ҺpH������ֽ������ɫʱ��˵����������������
(5)�������ӷ�����ȼ��������ʹ��ʧȥ֪�������������������ʱҪ��ˮԡ�Ͻ��������м�ֱ��������ȣ�Cװ����ƽ��ѹǿ����ȫ������ʩ����ˮ�����������ѡ��Cװ�ñȽ����ˣ�
(6)0.04mol����ૼ�ȩ���ۿ��Ƶ�0.02mol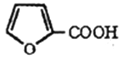 ������Ϊ0.02mol��112g/mol=2.24g��ૼ���IJ���
������Ϊ0.02mol��112g/mol=2.24g��ૼ���IJ���![]() ��100%=78.1%��
��100%=78.1%��