��Ŀ����
ijͬѧ������֪���ʵ���Ũ�ȵ�HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ������ش��������⣺
��1����ͬѧ��ʵ������������£�
A.ȡһ֧��ʽ�ζ��ܣ�������ˮϴ��
B.��������NaOH��Һ����¼Һ��̶ȶ���
C.����ʽ�ζ��ܾ�ȷ�ų�һ������Һ������δ����Һ��ϴ�Ľྻ����ƿ��
D.����ƿ�м���2�η�̪��Һ
E.�ζ�ʱ���ߵμӱ���ͬʱ��Ҫע�ӵζ�����Һ��ı仯
F.��С�ĵε���Һ����ɫ��ɷۺ�ɫʱ��30s�ڲ���ɫ��ֹͣ�ζ�
G.��¼Һ��̶ȶ���
H.�ݵζ��ܵ����ζ����ó�����NaOH��Һ�����Ϊ22mL
����ʵ��������д������__________�����ţ���ͬ����
��2�����в�������ʹ����NaOH��ҺŨ��ƫ�͵���___________��
A.��ʽ�ζ���ϴ��������HCl��Һ��ϴ��ֱ��ʹ��
B.�ζ�ǰ��ƿ������ˮϴ����δ������
C.��ʽ�ζ���©Һ
D.�Լ�ʽ�ζ��ܶ���ʱ����ʼʱƽ�ӣ�����ʱ����
E.�ζ������У���������ƿ���ڼ��ң�ʹ������Һ����ƿ��
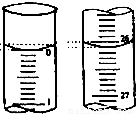
��3����һͬѧ��0.2010mol/L������ζ������ռ���Һ�������±����ݣ���������ռ���Һ��Ũ��Ϊ_______��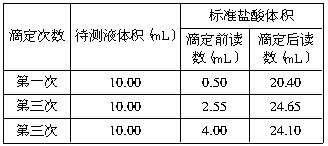
��4������ʱ������0.1mol/L��NaOH��Һ�ζ�25mL0.1mol/L�����ᣬ���յ�ʱ�����ٵμ���һ�Σ�ÿһ����ҺԼ0.05mL��NaOH��Һ���յ�ʱ��Һ��pH=_____����������μ���һ��NaOH��Һ���յ�ʱ��Һ��pH=_______��
���������
�����������
�����漰����к͵ζ��IJ�������������ʵ�����ݴ��������ʵ���Ũ�Ⱥ�pH�ļ����֪ʶ���ۺ��Խ�ǿ�����ʱ��Ҫȫ�濼�ǣ�������������ܵõ���ȷ�Ĵ𰸡�
��1������к͵ζ��IJ����У�������ʽ�ζ��ܺͼ�ʽ�ζ����ڵζ�ǰһ��Ҫϴ�ӣ�ϴ��ʱ����������ˮϴ�����ñ�Һ�����Һ��ϴ��װ����Һ����ƿҲҪϴ�ӣ���ֻ��������ˮϴ�������ô���Һ��ϴ�������ʹ����ƫ�ߣ�����ֵƫ���ڵζ������У���������ƿ�������յζ��ܵĻ������۾�ע����ƿ����Һ����ɫ�仯�͵μӵĿ���������ʱ����Ӧ���ζ�����Һ��İ�Һ����һ��ֱ���ϣ�����Ӧ������λС�������ݷ��������ղ������̣�����IJ�����B��E��H��
��2���к͵ζ�ʱ������ȷ�IJ������Т�c��NaOH��=V��HCl��?c��HCl��/V��NaOH����
���б�����������ƿ�У�δ֪Ũ�ȵ�NaOH��Һ���ڼ�ʽ�ζ����У��볣��ζ��෴��A��ʹ������ϡ�ͣ�����NaOH��Һ�������С����V��NaOH�����٣�c��NaOH��ƫ��B��������ˮϴ��δ���ˮ�Ĵ��ڶ�n��H+����Ӱ�죬��ˣ��Բⶨ���Ҳ��Ӱ�죻C����©Һ����V��HCl��ƫ��ʵ������V��NaOH������С��c��NaOH��ƫ��D��Լ�ʽ�ζ��ܶ���ʱ����ʼʱƽ�ӣ�����ʱ���ӣ�������ȡ��NaOH��Һ�����ƫ����c��NaOH��ƫС��E�ʹ��HCl��Һ���٣��ζ�ʱ��ȥNaOH��Һ�������С����c��NaOH��ƫ�ʴ�ΪD��
��3���ӱ������ݿɵã�V��HCl��1=19.90mL��V��HCl��2=22.10mL��V��HCl��3=20.10mL�������õ���������ڶ���������һ�Ρ�����������������ϴ���˵ڶ������ݲ�������ȡ��һ�κ͵��������ݽ��м��㣬V��HCl��ƽ��=��19.90mL+20.10mL��/2=20.00mL�����루2���Т�ʽ���ã�c��NaOH��=0.4020mol/L��
��4�����ٵμ�һ��NaOH��Һʱ���൱�ڶ�μ�һ�����ᣬ��ʱ��Һ��c��H+��=0.05��10-3��0.1/��25+25����10-3=1��10-4��mol/L����pH=-lg{c��H+��}=-lg��1��10-4��=4������μ�һ��NaOH��Һʱ�����������ʱ��Һ��c��OH-��=0.05��10-3��0.1/��25+25����10-3=1��10-4��mol/L����c��H+��=1��10-14/1��10-4=1��10-10��mol/L����pH=-lg��1��10-10��=10��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���֪������������ճ������м�Ϊ�������ᣬ��һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH ![]() CH3COO��+H+ ����H>0
CH3COO��+H+ ����H>0
��1��25 ��ʱ��Ũ�Ⱦ�Ϊ0.1mol/L������ʹ�����Һ������˵����ȷ���� ��
������Һ��pH��ͬ
������Һ�ĵ���������ͬ
����ˮ�������c(OH��)��ͬ
 ���к͵����ʵ�����NaOH��Һ����������Һ�������ͬ
���к͵����ʵ�����NaOH��Һ����������Һ�������ͬ
��2��25 ��ʱ����pH��Ϊ1������ʹ�����Һ�зֱ��ˮ�����ˮ
�������࣬����ҺpH�ı仯��ͼ��ʾ�����������pH�仯
�������� ��
��3��25 ��ʱ�������ΪVa mL pH=3�Ĵ�����Һ�еμ�pH=11��
NaOH��ҺVb mL����Һǡ�ó����ԣ���Va Vb���������������������
�����ʼ첿�Ź涨���۴���Ũ�Ȳ��õ���4.8g/100mL��ijͬѧ�����к͵ζ��ķ������ⶨijƷ�Ƶ�ʳ�ô��еĴ��Ậ���Ƿ��ꡣʵ����岽�����£���������ƽ��ȡһ������NaOH�����Ƴ�500mL NaOH��Һ��������֪Ũ�ȵ��������Һȷ�궨��NaOH��Һ��Ũ�ȣ�����������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��4����ֱ�������õ�NaOH��Һ�ζ���Ʒ����Ҫ�ñ������ȱ궨�ٵζ���ԭ���� ��
��5����ʵ��������£�ȷ��ȡ��ʳ�ô�20.00mL������250mL��ƿ�У��ٵμӷ�ָ̪ʾ�����ñ궨�õ�0.1000mol/L��NaOH��Һ�ζ�����ָ̪ʾ���� ɫǡ�ñ��__________ɫ�� ��Ϊ�յ㡣
�ظ��ζ���Σ������¼���£�
| �ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| V��mL�� | 19.40 | 15.10 | 14.90 | 15.00 |
���ʳ�ô��д�������ʵ���Ũ�ȣ�________ mol��L��1���Ƿ�ϸ� ����ǡ���
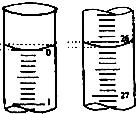 ijͬѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ������ش��������⣺
ijͬѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ������ش��������⣺