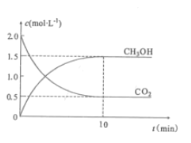
��Ŀ����
����Ŀ��ijѧ����0.10 mol��L��1��NaOH��Һ�ζ�ijŨ�ȵ����ᡣ��¼�������£�
ʵ�� ��� | ����Һ ���/mL | ������NaOH��Һ�����/mL | |
�ζ�ǰ����/mL | �ζ������/mL | ||
1 | 20.00 | 0.50 | 20.54 |
2 | 20.00 | 6.00 | 26.00 |
3 | 20.00 | 1.40 | 21.36 |

��1���ζ�ʱѡ�÷�̪��Һ��ָʾ��������жϵζ��ﵽ�յ�____________��
��2���ζ������У��۾�Ӧע��_____________________��
��3����������ʵ���Ũ��Ϊ___________��
��4����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���Բⶨ�����Ӱ����__________(����ƫ������ƫ����������Ӱ����)��
��5��ijͬѧ����֪ȷŨ�ȵĸ��������Һ�ζ���Һ��Fe2����Ũ�ȣ����������ҺӦʢ����________(����������������)�У��÷�Ӧ�����ӷ���ʽΪ_______________
���𰸡����������һ��NaOH��Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ�� ��ƿ����Һ��ɫ�ı仯�� 0.10 mol��L��1�� ƫ�� �� 5Fe2����MnO4-��8H��===5Fe3����Mn2����4H2O
��������
��1������Ϊ����Һ����������Ϊ��Һ��ָʾ��Ϊ��̪���ζ��յ�Ϊ��ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ������������������Ƶķ�Ӧ�������м��㣻
��2�����ݸ��������Һ����ǿ�����ԣ�Ӧ����ʽ�ζ���ʢ�ŷ�����
��3������ʵ�����ݵ���Ч�Խ��д�����
��4����ϲ�������������Ũ�ȵ�Ӱ�������
(1)��ƿ��Ϊ����ͷ�̪��Һ���ζ��յ�ı仯Ϊ�����������һ��NaOH��Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
(2)�ڵζ��������۾��۲���ƿ����Һ��ɫ�ı仯��
(3)���εζ���Ҫ������������Һ������ֱ�Ϊ��20.54-0.50=20.04mL��,2.00-6.00=20.00 mL��21.36-1.4=19.96 mL�����ε�ƽ��ֵΪ20.00 mL�������Ũ��Ϊ![]() = 0.10 mol��L��1��
= 0.10 mol��L��1��
(4) ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��������������Һ����������ⶨ�������Ũ��ƫ�ߣ�
(5) ���������Һ����ǿ�����ԣ���������ʽ�ζ���ʢ�ţ�ѡ��ף�������غ��������ӷ�Ӧ���������Ӻ������Ӻ�ˮ�����ӷ���ʽΪ��5Fe2����MnO4-��8H��===5Fe3����Mn2����4H2O��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�����Ŀ��N2O5��һ��������������һ���¶��·���2N2O5(g)![]() 4NO2(g)��O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ
4NO2(g)��O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ
t/s | 0 | 500 | 1 000 | 1 500 |
c(N2O5)mol/L | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ������ ��
A. 500 s��N2O5�ֽ�����Ϊ2.96��10��3 mol/(L��s)
B. T1�¶��µ�ƽ�ⳣ��ΪK1��125��1 000 sʱת����Ϊ50%
C. ������������ʱ��T2�¶��·�Ӧ��1 000 sʱ���N2O5(g)Ũ��Ϊ2.98 mol/L����T1<T2
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1>K2