��Ŀ����
ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
��1������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ����Ŀ����___________��
��2������ƿ�м���һ���������Ҵ���Ũ����Ļ��Һ�ķ����ǣ� ��
��3���ڸ�ʵ���У�����1mol�Ҵ���1mol ������Ũ���������¼��ȣ���ַ�Ӧ���ܷ�����1mol���������� ��ԭ���� ��
��4��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a��__________���Լ�b��_______________�����뷽������__________�����뷽������__________________�����뷽������_______________��
��5���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ����
��
��6��д��C �� D ��Ӧ�Ļ�ѧ����ʽ ��
��1����ֹ��ƿ��Һ�屩�У�1�֣�
��2��������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ�����2�֣�
��3���÷�Ӧ�ǿ��淴Ӧ����Ӧ���ܽ��е��ף�1+2�֣�
��4������̼������Һ��Ũ���ᣬ��Һ���������� ����1�֣�
��5����ȥ���������л��е�����ˮ��1�֣�
��6��2CH3COONa + H2SO4 = Na2SO4 + 2CH3COOH��2�֣�
���������������1����Ӧ��Ҫ���ȣ�����뼸�����Ƭ��Ŀ���Ƿ�ֹ��ƿ��Һ�屩�С�
��2��Ũ������ܶȴ���ˮ�ģ�������ˮ���ȣ����Ҵ������ᶼ���ӷ��ģ���������ƿ�м���һ���������Ҵ���Ũ����Ļ��Һ�ķ�����������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
��3�����ڸ÷�Ӧ�ǿ��淴Ӧ����Ӧ���ܽ��е��ף����Բ�������1mol����������
��4���������ɵ����������к���������Ҵ������Լ�a�DZ���̼������Һ����Һ����ʵ�����������ķ��룬A���T�õ���������������F������������B�к��������ƺ��Ҵ��Լ�̼���ƣ���ͨ�����õ��Ҵ�������E���Ҵ���C�к��������ƺ�̼���ƣ������ϡ���ἴ�������ᣬȻ������õ����ᡣ
���㣺���������������Ʊ������ʵķ�����ᴿ
�������������е��Ѷȵ����⣬������۽̲Ļ���֪ʶ��ּ�ڹ���ѧ���Ļ��������ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶��ʵ��������������ѧ����Ӧ��������

 ��У����ϵ�д�
��У����ϵ�д�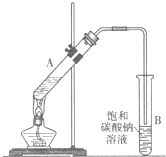 ������������Ҫ�Ļ���ԭ�ϣ�ʵ���Һϳ�����������װ����ͼ��ʾ��
������������Ҫ�Ļ���ԭ�ϣ�ʵ���Һϳ�����������װ����ͼ��ʾ���й����ݼ�����Ӧ��
| ���� | �Ҵ� | �������� | ���� | |
| �е�/�� | 118 | 78.3 | 77.1 | 34.5 |
| �ܽ��� | ������ˮ | ��������ˮ | �����ѻ��� | ����ˮ |
| Ũ���� |
| ��140 |
��ش��������⣺
��1���ڴ��Թ�A�����ӵ��Լ���6mL�Ҵ���4mL�����4mLŨ���ᣬ�������Լ�������˳������Ϊ
��2���Թ�B�е��ܽӽ�Һ��δ����Һ���µ�������
��3���ֶ��Թ�B�����������ֲ�Ʒ�����ᴿ���������£�
�ٽ��Թ�B�л��Һ������ת��
�����������ϲ�Һ���м�����ˮ�����ƣ������������ˮ�����Ƶ�Ŀ���ǣ�
�۽���������������Һ���������������ƿ�У�������������ռ�
��4������ɫ��ѧ�ĽǶȷ�����ʹ��Ũ������������������֮����Ҫ��
��5������ʱ����һ��ƺʹ���ʹ��������ɿڣ����÷���ʵ������Ļ�ѧ����ʽ���ͣ�

 ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩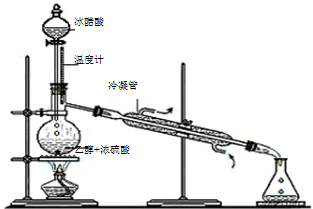 �л���ѧ֪ʶ��������Ӧ�ù㷺��
�л���ѧ֪ʶ��������Ӧ�ù㷺��