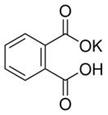
��Ŀ����
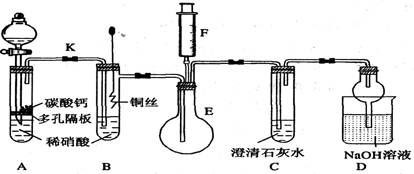
(��10��)��ѧ�ϳ���ȼ�շ�ȷ���л������ɣ����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɣ���ͼ����װ������ȼ�շ�ȷ���л������ʽ���õ�װ�ã�

�ش��������⣺
��1��������������������������ѡװ�ø����ܵ�������ţ���д�ӿڴ��ţ��ǣ�
________________________________________��
��2��Cװ����ŨH2SO4��������________________________________��
��3��Dװ����MnO2��������__________________________________��
��4��ȼ�չ���CuO��������___________________________________��
��5����ȷ��ȡ 0.90 g��Ʒ��ֻ�� C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�
A����������1.32 g��B����������0.54 g������л�������Ԫ�ص�����Ϊ___________��

�ش��������⣺
��1��������������������������ѡװ�ø����ܵ�������ţ���д�ӿڴ��ţ��ǣ�
________________________________________��
��2��Cװ����ŨH2SO4��������________________________________��
��3��Dװ����MnO2��������__________________________________��
��4��ȼ�չ���CuO��������___________________________________��
��5����ȷ��ȡ 0.90 g��Ʒ��ֻ�� C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�
A����������1.32 g��B����������0.54 g������л�������Ԫ�ص�����Ϊ___________��
(1)g��f��e��h��i��c��d��a��b����2������ˮ�֣��ø��﴿����O2 ��3��������4��ʹ�л�����������CO2��H2O ��5��CH2O
�����л������ʽ��ȷ����
Dװ�������������ģ����ö������̵Ĵ�����ʹ˫��ˮ�ֽ�������������ڲ��뷴Ӧ�����������Ǵ�������ģ�������Ҫ�����ɵ��������о�����������Ũ�������������Ȼ����л��ﷴӦ���л����ȼ�ղ�������Ӧ��������ˮ�Ȼ������������ɵ�ˮ��Ȼ���������������������ɵ�CO2���л�����ȼ�չ�����Ҳ����ȼ�ղ���֣��Ӷ�����CO�������Ҫ������ͭ�������������ɵ�CO��A����������1.32 g����CO2��������1.32g�����ʵ�����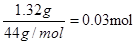 ��B����������0.54 g����ˮ��0.54g�����ʵ�����
��B����������0.54 g����ˮ��0.54g�����ʵ�����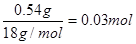 �������л�������ԭ�ӵ�������0.90g��0.03mol��12g/mol��0.06mol��1g/mol��0.48mol�����ʵ�����0.03mol������C��H��O�ĸ���֮����1�U2�U1��������ʽ��CH2O��
�������л�������ԭ�ӵ�������0.90g��0.03mol��12g/mol��0.06mol��1g/mol��0.48mol�����ʵ�����0.03mol������C��H��O�ĸ���֮����1�U2�U1��������ʽ��CH2O��
Dװ�������������ģ����ö������̵Ĵ�����ʹ˫��ˮ�ֽ�������������ڲ��뷴Ӧ�����������Ǵ�������ģ�������Ҫ�����ɵ��������о�����������Ũ�������������Ȼ����л��ﷴӦ���л����ȼ�ղ�������Ӧ��������ˮ�Ȼ������������ɵ�ˮ��Ȼ���������������������ɵ�CO2���л�����ȼ�չ�����Ҳ����ȼ�ղ���֣��Ӷ�����CO�������Ҫ������ͭ�������������ɵ�CO��A����������1.32 g����CO2��������1.32g�����ʵ�����
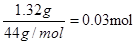 ��B����������0.54 g����ˮ��0.54g�����ʵ�����
��B����������0.54 g����ˮ��0.54g�����ʵ�����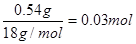 �������л�������ԭ�ӵ�������0.90g��0.03mol��12g/mol��0.06mol��1g/mol��0.48mol�����ʵ�����0.03mol������C��H��O�ĸ���֮����1�U2�U1��������ʽ��CH2O��
�������л�������ԭ�ӵ�������0.90g��0.03mol��12g/mol��0.06mol��1g/mol��0.48mol�����ʵ�����0.03mol������C��H��O�ĸ���֮����1�U2�U1��������ʽ��CH2O��
��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д�
�����Ŀ