��Ŀ����
�� H2��O2�������� Na2CO3 ���ȼ�ϵ�أ����õ�ⷨ�Ʊ� Fe(OH)2��װ������ͼ��ʾ������ P��ͨ��CO2��ͨ��һ��ʱ����Ҳಣ�����в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ��������˵������ȷ����

A��X��Y ���˶������������缫
B���������� NaOH ��Һ��Ϊ���Һ
C�����������ķ�Ӧ�ǣ�2H2O��2e��=H2��+ 2OH-
D��X ��Ϊ���ص�����
��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ
25��ʱ������ͬŨ�ȵĶ�Ԫ����H2A��NaOH��Һ��������(����仯���Բ���)���跴Ӧ����Һ��pH���±���
ʵ���� | ��ʼŨ��/mol��L��1 | ��Ӧ����Һ��pH | |
c(H2A) | c | ||
�� | x | 0.10 | 9 |
�� | 0.10 | 0.10 | 5 |
�����жϲ���ȷ����
A��x��0.10
B��HA���ĵ���̶ȴ���ˮ��̶�
C��ʵ���������Һ��c(Na+)��c(A2��)+c(HA��)+c(H2A)
D����ʵ���������Һ��ˮϡ�ͺ�c(A2��)/c(HA��)���


 ���вⶨ��
���вⶨ��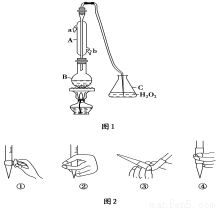
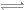 2NH3(g) ��H=-93.0kJ/mol
2NH3(g) ��H=-93.0kJ/mol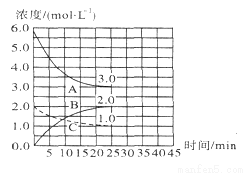
 ��Ӧ������ ��
��Ӧ������ �� 5N2(g)+6H2O(g) ��H<0
5N2(g)+6H2O(g) ��H<0
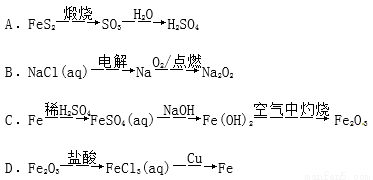
 (NaOH)
(NaOH)