��Ŀ����
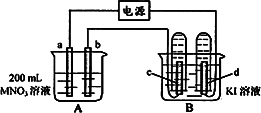
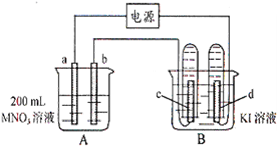
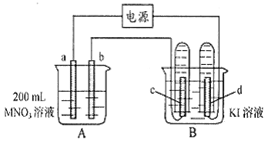
����ͼ��ʾװ���У�b�缫�ý���M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�
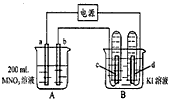
��1��aΪ_________����c���ĵ缫��ӦʽΪ��________________��
��2����ʼʱ����B�ձ�������μ��ε�����Һ�����ܹ۲쵽��������________________��������һ��ʱ�������c���ϵ��Թ���Ҳ�ռ��������壬��ʱc���ϵĵ缫��ӦΪ��________________��
��3����d�缫���ռ���44��8ml���壨��״����ʱֹͣ��⣬��b�缫�ϳ�������M������Ϊ0��432g����˽�����Ħ������Ϊ________________��
��4�����ֹͣ�������ˮʹA�ձ��е���Һ�����Ϊ200 ml��ȡ������Һ���뵽25��0 ml 0��100 mol��L-1
��HCl��Һ�У�������31��25 ml ��Һʱ�պó�����ȫ���ɴ˿�֪���ǰA�ձ���MNO3��Һ�����ʵ���Ũ��Ϊ________mol��L-1��
��2����ʼʱ����B�ձ�������μ��ε�����Һ�����ܹ۲쵽��������________________��������һ��ʱ�������c���ϵ��Թ���Ҳ�ռ��������壬��ʱc���ϵĵ缫��ӦΪ��________________��
��3����d�缫���ռ���44��8ml���壨��״����ʱֹͣ��⣬��b�缫�ϳ�������M������Ϊ0��432g����˽�����Ħ������Ϊ________________��
��4�����ֹͣ�������ˮʹA�ձ��е���Һ�����Ϊ200 ml��ȡ������Һ���뵽25��0 ml 0��100 mol��L-1
��HCl��Һ�У�������31��25 ml ��Һʱ�պó�����ȫ���ɴ˿�֪���ǰA�ձ���MNO3��Һ�����ʵ���Ũ��Ϊ________mol��L-1��
��1������2I--2e-==I2
��2��C����������Һ���ȱ�Ϊ��ɫ��4OH-- 4e-==2H2O+O2��
��3��108g/mol
��4��0.1mol��L-1
��2��C����������Һ���ȱ�Ϊ��ɫ��4OH-- 4e-==2H2O+O2��
��3��108g/mol
��4��0.1mol��L-1

��ϰ��ϵ�д�
�����Ŀ