��Ŀ����
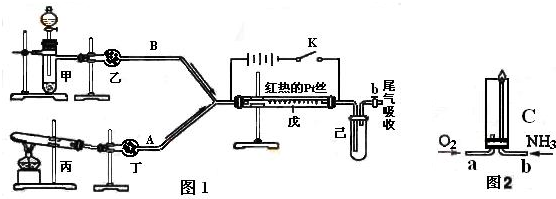
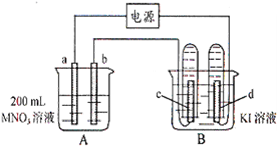
10�֣� ����ͼ��ʾװ���У�b�缫�ý���M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�

��1��aΪ ��
��2����ʼʱ����B�ձ�������μ��ε�����Һ�����ܹ۲쵽�������� ��������һ��ʱ�������c���ϵ��Թ���Ҳ�ռ��������壬��ʱc���ϵĵ缫��ӦΪ�� ��
��3����d�缫���ռ���44.8 ml���壨��״����ʱֹͣ��⣬a���Ϸų��� ml���壬��b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ g��moL-1��
1.�� 2.��C������ 4OH����4e��=2H2O+O2�� 3. 22.4 108
����������1������M������b���ϣ�˵��b����������a��������
��2�����ݣ�1����֪d����������Һ�е������ӷŵ����������� c����������Һ�еĵ����ӷŵ磬���ɵⵥ�ʣ�����c����Һ����ɫ����c���ϵ��Թ���Ҳ�ռ���������ʱ��˵����ʱ��Һ�е�OH����c���Ϸŵ磬��ӦʽΪ4OH����4e��=2H2O+O2����
��3��d���ռ����������������ʵ���Ϊ0.002mol��ת�Ƶ�����0.004mol�����Ը��ݵ��ӵĵ�ʧ�غ��֪��a������������22.4ml����������M�Ļ��ϼ��ǣ�1�ۣ��������ɽ���M�����ʵ�����0.004nol�������ԭ��������0.432��0.004��108��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�