��Ŀ����
��ͼ�Dz���Ԫ�صĵ��ʺͻ�����֮����ת����ϵ�����ַ�Ӧ��������û��ȫ���г�����֪ԭ��A��M�����ڽ��������L�������ֳ�����ɵġ�F��Y��Ӧ������һ��ȫ��Ϊ�ǽ���Ԫ������ɵ��Σ�C����ɫ��Ӧ�ʻ�ɫ��M�д��ԣ���Ӧ����B��C�����ʵ���֮��Ϊ3��4��

�ش���������
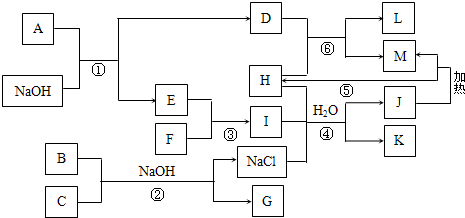
��1����Ӧ�ڵ���Ҫ��;�ǣ�_________________����һ�㼴�ɣ���Y�Ŀռ乹��Ϊ____________��
��2����֪��Ӧ���л���һ����ɫ�������ɣ�д����Ӧ�۵����ӷ���ʽ��________________________��
��3��L�����ֳ���Ϊ____________���û�ѧʽ��ʾ����A�Ļ�ѧʽΪ____________��
��4������M����ϡ�����п��γ���ҺZ������Ƽ�ʵ�飬֤��Z��Һ�������Ľ��������ӣ�____________________________________��
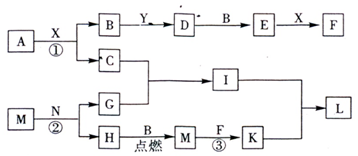
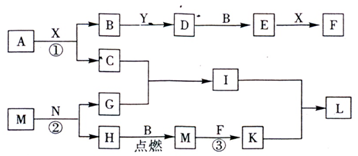
��1����Ӧ�ڵ���Ҫ��;�ǣ�_________________����һ�㼴�ɣ���Y�Ŀռ乹��Ϊ____________��
��2����֪��Ӧ���л���һ����ɫ�������ɣ�д����Ӧ�۵����ӷ���ʽ��________________________��
��3��L�����ֳ���Ϊ____________���û�ѧʽ��ʾ����A�Ļ�ѧʽΪ____________��
��4������M����ϡ�����п��γ���ҺZ������Ƽ�ʵ�飬֤��Z��Һ�������Ľ��������ӣ�____________________________________��
��1�����ԡ�
��2��3Fe3O4+28H++NO3-==9Fe3++NO+14H2O
��3��Fe(OH)3��Al(OH)3��NaO2
��4���ֱ�ȡ��������Һ����֧�ྻ���Թ��У����һ֧�Թ��еμ����軯����Һ����Һ��Ѫ��ɫ��֤����Fe3+����ڶ�ֻ�Թ��У��μ��������Ը��������Һ�������������ɫ��ȥ��֤������Fe2+
��2��3Fe3O4+28H++NO3-==9Fe3++NO+14H2O
��3��Fe(OH)3��Al(OH)3��NaO2
��4���ֱ�ȡ��������Һ����֧�ྻ���Թ��У����һ֧�Թ��еμ����軯����Һ����Һ��Ѫ��ɫ��֤����Fe3+����ڶ�ֻ�Թ��У��μ��������Ը��������Һ�������������ɫ��ȥ��֤������Fe2+

��ϰ��ϵ�д�
�����Ŀ