��Ŀ����
��2010?��ƽ��ģ�⣩�����������壨FeSO4?7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�

��ش��������⣺
��1�����֤���������Һ�к���Fe2+
��2������ڼ������H2O2��Ŀ�ģ�
��3��������з�Ӧ�����ӷ���ʽ��
��4���������һϵ�д����IJ������裺
��5����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����Ͳ��ҩ�ס���ͷ�ι��⣬����
������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
��6��������ÿ��Ӧ����14mg���ҵ��������о���������ʳ����ȫ��ͨ�����ú�FeSO4?7H2O��Ƭ��������������������ÿ������ú�

��ش��������⣺
��1�����֤���������Һ�к���Fe2+
ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ
ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ
����2������ڼ������H2O2��Ŀ�ģ�
��Fe2+ȫ������ΪFe3+
��Fe2+ȫ������ΪFe3+
����3��������з�Ӧ�����ӷ���ʽ��
Fe3++3OH-=Fe��OH��3������Fe3++3NH3?H2O=Fe��OH��3��+3NH4+��
Fe3++3OH-=Fe��OH��3������Fe3++3NH3?H2O=Fe��OH��3��+3NH4+��
����4���������һϵ�д����IJ������裺
����
����
��ϴ�ӡ����ա���ȴ
��ȴ
����������5����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����Ͳ��ҩ�ס���ͷ�ι��⣬����
250mL����ƿ
250mL����ƿ
��������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������
b
b
��a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
��6��������ÿ��Ӧ����14mg���ҵ��������о���������ʳ����ȫ��ͨ�����ú�FeSO4?7H2O��Ƭ��������������������ÿ������ú�
69.5mg
69.5mg
mg FeSO4?7H2O��Ƭ������������1���������Ӿ��л�ԭ�ԣ��ܱ�����������Ϊ�����ۣ����������軯�ؼ����������Ĵ��ڣ�
��2��˫��ˮ���������ԣ���ԭ������ˮ��
��3���������ͼӦ������������������
��4�������������ȶ������ֽ⣬������Һ�еij������ֽ����Ҫ������;�����ˡ�ϴ�ӡ����ա���ȴ��������
��5���ڸ�������һ�����ʵ���Ũ�ȵ���Һ����Ҫ���������ش�
�����ᡢ���������ǿ�����ԣ�
��6��������Ԫ���غ������㣮
��2��˫��ˮ���������ԣ���ԭ������ˮ��
��3���������ͼӦ������������������
��4�������������ȶ������ֽ⣬������Һ�еij������ֽ����Ҫ������;�����ˡ�ϴ�ӡ����ա���ȴ��������
��5���ڸ�������һ�����ʵ���Ũ�ȵ���Һ����Ҫ���������ش�
�����ᡢ���������ǿ�����ԣ�
��6��������Ԫ���غ������㣮
����⣺��1���������Ӿ��л�ԭ�ԣ��ܱ�����������Ϊ�����ۣ�������������������Ժ�ɫ���ʴ�Ϊ��ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ��
��2��˫��ˮ���������ԣ��ܽ�Fe2+ȫ������ΪFe3+���ʴ�Ϊ����Fe2+ȫ������ΪFe3+��
��3���������ͼӦ���������������ɫ���������Լ����XΪ��ʴ�Ϊ��Fe3++3OH-=Fe��OH��3������Fe3++3NH3?H2O=Fe��OH��3��+3NH4+����
��4��������Һ�н�������������Ҫ�ȹ��ˣ�Ȼ��Թ���ϴ�ӣ���ϴ�Ӹɾ��������շֽ⣬����ȴ��������ʴ�Ϊ�����ˣ���ȴ��
��5���ٸ�������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У�250mL����ƿ����ƽ���������ձ�����Ͳ��ҩ�ס���ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ��
�ڸ��������ǿ�����ԣ��ܽ�����������������������ԣ�������������������ֻ����ϡ�����ữ���ʴ�Ϊ��b��
��6��������Ԫ���غ㣬14mg������ΪFeSO4?7H2OƬ��������������������ҪFeSO4?7H2OƬ������=14mg��
=69.5mg���ʴ�Ϊ��69.5mg��
��2��˫��ˮ���������ԣ��ܽ�Fe2+ȫ������ΪFe3+���ʴ�Ϊ����Fe2+ȫ������ΪFe3+��
��3���������ͼӦ���������������ɫ���������Լ����XΪ��ʴ�Ϊ��Fe3++3OH-=Fe��OH��3������Fe3++3NH3?H2O=Fe��OH��3��+3NH4+����
��4��������Һ�н�������������Ҫ�ȹ��ˣ�Ȼ��Թ���ϴ�ӣ���ϴ�Ӹɾ��������շֽ⣬����ȴ��������ʴ�Ϊ�����ˣ���ȴ��
��5���ٸ�������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У�250mL����ƿ����ƽ���������ձ�����Ͳ��ҩ�ס���ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ��
�ڸ��������ǿ�����ԣ��ܽ�����������������������ԣ�������������������ֻ����ϡ�����ữ���ʴ�Ϊ��b��
��6��������Ԫ���غ㣬14mg������ΪFeSO4?7H2OƬ��������������������ҪFeSO4?7H2OƬ������=14mg��
| 56 |
| 278 |
������������һ������������Ļ��������ʵ��ۺ���֪ʶ��Ŀ��Ҫ��ѧ�����з����ͽ�������������

��ϰ��ϵ�д�
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
�����Ŀ
��2010?��ƽ��ģ�⣩�±��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ���ǣ�������
|
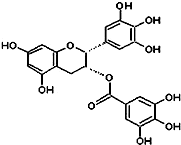 ��2010?��ƽ��ģ�⣩�̲��к���EGCG����ʾûʳ�Ӷ�����ûʳ�����������ʾ��п������ã���֪EGCG��һ��������A��ͼ��ʾ���й�������A˵������ȷ���ǣ�������
��2010?��ƽ��ģ�⣩�̲��к���EGCG����ʾûʳ�Ӷ�����ûʳ�����������ʾ��п������ã���֪EGCG��һ��������A��ͼ��ʾ���й�������A˵������ȷ���ǣ������� CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011
CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011 CH3CH2OH��g��+H2O��g�� 25��ʱ��K=1.71��1022
CH3CH2OH��g��+H2O��g�� 25��ʱ��K=1.71��1022