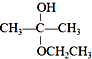��Ŀ����
����Ŀ��ʵ�����ù����ռ�����480mL0.1000mol/L��NaOH��Һ��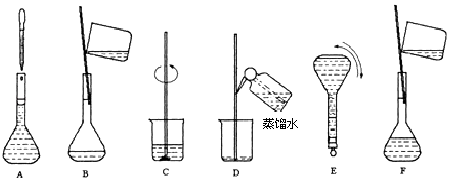
��1�������g�ռӦ���������������ƣ��г���
��2�����ƹ����У�����Ҫ����������д���ţ�
a���ձ� b����Ͳ c�������� d��1000mL����ƿ e��©�� f����ͷ�ι�
��3������ʵ����Ҫ�ͣ�2�����������жϣ����ʵ�黹ȱ�ٵ������� �� ����ҩ��
��4������Bͨ����Ϊת�ƣ�����Aͨ����Ϊ
��5��������ʵ�鲽��A��F��ʵ������Ⱥ��������
��6��ʵ�������õ��������������÷ֱ��ǣ�����������������
��7��������������������н�����������ҺŨ��ƫ�ߵ����������б�ţ�
������ƿʵ��ǰ������ˮϴ�ɾ�����δ���
�ڶ��ݹ۲�Һ��ʱ����
�����ƹ�������©�ˣ�4���в���D
�ܼ�����ˮʱ���������˿̶�
��8����ʵ������г��֣�7���Т���������㽫��δ����� ��
���𰸡�
��1��2.0,С�ձ�
��2��de
��3��������ƽ,500mL����ƿ,С�ձ�
��4������
��5��CBDFAE
��6������,����
��7����
��8����������
���������⣺��1������ʵ������480mL����ƿ����Ӧѡ��500mL����ƿ�����Ƴ�500mL��Һ�������m=nM=cVM��֪��Ҫ�������Ƶ�����m=0.1mol/L��0.5L��40g/mol=2.0g���������ƹ���ij�����Ҫ��С�ձ��н��У�
���Դ��ǣ�2.0��С�ձ���
��2������ʵ�鲽��Ϊ�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��Ҫ��������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܡ���Ͳ�����ÿɲ��ã���500mL�Լ�ƿ���ò���������Ϊ��1000mL����ƿ��©����
���Դ��ǣ�de��
��3�����ݣ�2����֪ȱ�ٵ�������500mL����ƿ��������ƽ��С�ձ���
���Դ��ǣ�������ƽ��500mL����ƿ��С�ձ���
��4������AΪ���ݣ��ý�ͷ�ι���εμ�����ˮ����Һ����ʹ���̶������У�
���Դ��ǣ����ݣ�
��5������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ���������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������ȷ�IJ���˳��Ϊ��CBDFAE��
���Դ��ǣ�CBDFAE��
��6�����������ܽ����ʱ���ڽ�����ٹ����ܽ⣻��Һʱ��Ӧ������������
���Դ��ǣ����裻������
��7��������ƿʵ��ǰ������ˮϴ�ɾ�����δ��ɣ������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
�ڶ��ݹ۲�Һ��ʱ���ӣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
�����ƹ�������©�ˣ�4���в���D�����²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�ܼ�����ˮʱ���������˿̶ȣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
���Դ��ǣ��ڣ���8����ˮ����������ʵ��ʧ���Ҳ��ܲ��ȣ���Ҫ�������ƣ�
���Դ��ǣ��������ƣ�
�����㾫������������һ�����ʵ���Ũ�ȵ���Һ�ǽ����ĸ�������Ҫ֪���������ʵ���Ũ����Һʱ�������ձ�������ˮ������ƿ�̶���1cm��2cm���ٸ��ý�Ͷ�ιܼ�ˮ���̶��ߣ�