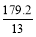ЬтФПФкШн
аТЕФЁЖЛЗОГПеЦјжЪСПБъзМЁЗНЋгк2016Фъ1дТ1ШедкЮвЙњШЋУцЪЕЪЉЁЃОнДЫЃЌЛЗОГПеЦјжЪСПжИЪ§(AQI)ШеБЈКЭЪЕЪББЈИцАќРЈСЫSO2ЁЂNO2ЁЂCOЁЂO3ЁЂPM10ЁЂPM2.5ЕШжИБъЃЌЮЊЙЋжкЬсЙЉНЁПЕжИв§ЃЌв§ЕМЕБЕиОгУёКЯРэАВХХГіааКЭЩњЛюЁЃ
(1)ЦћГЕХХГіЕФЮВЦјжаКЌгаCOКЭNOЕШЦјЬхЃЌгУЛЏбЇЗНГЬЪННтЪЭВњЩњNOЕФдвђ________________________________________
(2)ЦћГЕХХЦјЙмФкАВзАЕФДпЛЏзЊЛЏЦїЃЌПЩЪЙЦћГЕЮВЦјжаЕФжївЊЮлШОЮязЊЛЏЮЊЮоЖОЕФДѓЦјбЛЗЮяжЪЁЃвбжЊЃК
N2(g)ЃЋO2(g)===2NO(g)ЁЁІЄHЃНЃЋ180.5 kJЁЄmolЃ1
2C(s)ЃЋO2(g)===2CO(g)ЁЁІЄHЃНЃ221.0 kJЁЄmolЃ1
C(s)ЃЋO2(g)===CO2(g)ЁЁІЄHЃНЃ393.5 kJЁЄmolЃ1
дђЗДгІ2NO(g)ЃЋ2CO(g)??N2(g)ЃЋ2CO2(g)ЕФІЄHЃН________kJЁЄmolЃ1ЃЛИУЗДгІЕФІЄS________0(ЬюЁА>ЁБЁА<ЁБЛђЁАЃНЁБ)ЁЃ
(3)НЋ0.20 mol NOКЭ0.10 mol COГфШывЛИіШнЛ§КуЖЈЮЊ1 LЕФУмБеШнЦїжаЃЌдкВЛЭЌЬѕМўЯТЗДгІЙ§ГЬжаВПЗжЮяжЪЕФХЈЖШБфЛЏзДПіШчЭМЫљЪОЁЃ
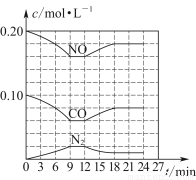
ЂйМЦЫуВњЮяN2дк6ЁЋ9 minЪБЕФЦНОљЗДгІЫйТЪv(N2)ЃН________molЁЄLЃ1ЁЄminЃ1ЃЛ
ЂкЕк12 minЪБИФБфЕФЗДгІЬѕМўЮЊ________(ЬюЁАЩ§ЮТЁБЛђЁАНЕЮТЁБ)ЃЛ
ЂлМЦЫуЗДгІдкЕк24 minЪБЕФЦНКтГЃЪ§KЃН________ЁЃШєБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаГфШыCOЁЂN2Иї0.060 molЃЌЦНКтНЋ________вЦЖЏ(ЬюЁАе§ЯђЁБЁАФцЯђЁБЛђЁАВЛЁБ)ЁЃ
(4)ЛЗОГМрВтжаЛЙПЩгУГСЕэЗЈВтЖЈПеЦјжаКЌгаНЯИпХЈЖШSO2ЕФКЌСПЃЌОВщЕУвЛаЉЮяжЪдк20 ЁцЕФЪ§ОнШчЯТБэЃК
ШмНтЖШ(S)/g | ШмЖШЛ§(Ksp) | ||
Ca(OH)2 | Ba(OH)2 | CaSO3 | BaSO3 |
0.160 | 3.89 | 6.76ЁС10Ѓ3 | 5.48ЁС10Ѓ9 |
ЂйЮќЪеSO2зюКЯЪЪЕФЪдМСЪЧ________[ЬюЁАCa(OH)2ЁБЛђЁАBa(OH)2ЁБ]ШмвКЃЛ
Ђкдк20 ЁцЪБЃЌЯђCaSO3аќзЧвКжаЕЮМгЪЪСПЕФBaCl2ШмвКЃЌЕБCaSO3ЯђBaSO3ЕФзЊЛЏДяЕНЦНКтЪБЃЌШмвКжаЕФ 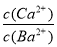 ЃН____________(аДГіБэДяЪНМДПЩ)ЁЃ
ЃН____________(аДГіБэДяЪНМДПЩ)ЁЃ
(1)N2ЃЋO2 2NO(2)Ѓ746.5ЁЁ<(3)Ђй3.3ЁС10Ѓ3ЂкЩ§ЮТЂл0.019ЁЁФцЯђ(4)ЂйBa(OH)2
2NO(2)Ѓ746.5ЁЁ<(3)Ђй3.3ЁС10Ѓ3ЂкЩ§ЮТЂл0.019ЁЁФцЯђ(4)ЂйBa(OH)2
Ђк 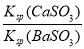 Лђ
Лђ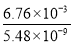
ЁОНтЮіЁП(1)ПеЦјжаЕФЕЊЦјКЭбѕЦјдкЦћГЕЗЂЖЏЛњФкЕчЛ№ЛЈв§ШМЯТЃЌЗЂЩњЗДгІЩњГЩNOЁЃ(2)НЋвбжЊЕФШ§ИіШШЛЏбЇЗНГЬЪНвРДЮБрКХЮЊЂйЁЂЂкЁЂЂлЃЌИљОнИЧЫЙЖЈТЩЂлЁС2ЃЂкЃЂйЃЌМДПЩЧѓГіФПБъШШЛЏбЇЗНГЬЪНЕФІЄHЁЃИУШШЛЏбЇЗНГЬЪНБэЪОЕФЗДгІЪЧЦјЬхЬхЛ§МѕаЁЕФЗДгІЃЌьиБфаЁгк0ЁЃ(3)Ђйv(N2)ЃН0.01/3 ЃН3.3ЁС10Ѓ3 molЁЄLЃ1ЁЄminЃ1ЁЃЂкгЩЭМжаПДГіЕк12 minЗДгІЯђФцЗДгІЗНЯђвЦЖЏЃЌЮЊЩ§ИпЮТЖШЫљжТЁЃЂлЕк24 minЪБCO2ЕФХЈЖШЮЊ0.02 molЁЄLЃ1ЃЌЙЪЦНКтГЃЪ§KЃН0.019ЁЃгЩЦНКтГЃЪ§ЕФБэДяЪНПДГіЃЌШєCOЁЂN2ЭЌЪБдіМг0.06 molЃЌХЈЖШЩЬЛсДѓгкЦНКтГЃЪ§ЃЌЦНКтФцЯђвЦЖЏЁЃ(4)ЂйгЩБэжаЪ§ОнПДГіBa(OH)2ШмНтЖШДѓЃЌЯрЭЌЬхЛ§ЕФЮќЪевКЃЌЦфЮќЪеSO2ЕФСПЖрЃЌЧвBaSO3ШмНтЖШИќаЁЃЌБугкSO32ЁЊНЯЭъШЋГСЕэЮіГіЃЌМѕаЁЪЕбщЮѓВюЁЃЂкШмвКжаДцдкЦНКтCaSO3ЃЋBa2ЃЋ BaSO3ЃЋCa2ЃЋЃЌШмвКжаЕФвѕРызгЮЊSO32ЁЊЃЌЙЪ
BaSO3ЃЋCa2ЃЋЃЌШмвКжаЕФвѕРызгЮЊSO32ЁЊЃЌЙЪ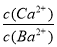 ЃН
ЃН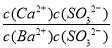 ЃН
ЃН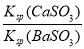

баОПCO2ЕФРћгУЖдДйНјЕЭЬМЩчЛсЕФЙЙНЈОпгаживЊЕФвтвхЁЃ
(1)НЋCO2гыНЙЬПзїгУЩњГЩCOЃЌCOПЩгУгкСЖЬњЕШЁЃ
ЂйвбжЊЃКFe2O3(s)ЃЋ3C(ЪЏФЋ)=2Fe(s)ЃЋ3CO(g)ЁЁІЄH1ЃНЃЋ489.0 kJЁЄmolЃ1
C(ЪЏФЋ)ЃЋCO2(g)=2CO(g)ЁЁІЄH2ЃНЃЋ172.5 kJЁЄmolЃ1
дђCOЛЙдFe2O3ЕФШШЛЏбЇЗНГЬЪНЮЊ___________________________
ЂкРћгУШМЩеЗДгІПЩЩшМЦГЩCO/O2ШМСЯЕчГи(вдKOHШмвКЮЊЕчНтвК)ЃЌаДГіИУЕчГиЕФИКМЋЗДгІЪН___________________________________________
(2)ФГЪЕбщНЋCO2КЭH2ГфШывЛЖЈЬхЛ§ЕФУмБеШнЦїжаЃЌдкСНжжВЛЭЌЬѕМўЯТЗДгІЃК
CO2(g)ЃЋ3H2(g) CH3OH(g)ЃЋH2O(g)ЁЁ ІЄHЃНЃ49.0 kJЁЄmolЃ1
CH3OH(g)ЃЋH2O(g)ЁЁ ІЄHЃНЃ49.0 kJЁЄmolЃ1

ВтЕУCH3OHЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЩЯЭМЫљЪОЃЌЛиД№ЮЪЬтЃК
ЂйЯТСаДыЪЉжаФмЪЙn(CH3OH)/n(CO2)діДѓЕФЪЧ________ЁЃ
AЃЎЩ§ИпЮТЖШ BЃЎГфШыHe(g)ЪЙЬхЯЕбЙЧПдіДѓ
CЃЎНЋH2O(g)ДгЬхЯЕжаЗжРы DЃЎдйГфШы1 mol CO2КЭ3 mol H2
ЂкЧњЯпЂёЁЂЂђЖдгІЕФЦНКтГЃЪ§ДѓаЁЙиЯЕЮЊKЂё________KЂђ(ЬюЁАДѓгкЁБЁАЕШгкЁБЛђЁАаЁгкЁБ)ЁЃ
ЂлвЛЖЈЮТЖШЯТЃЌдкШнЛ§ЯрЭЌЧвЙЬЖЈЕФСНИіУмБеШнЦїжаЃЌАДШчЯТЗНЪНЭЖШыЗДгІЮяЃЌвЛЖЮЪБМфКѓДяЕНЦНКтЁЃ
ШнЦї | Мз | вв |
ЗДгІЮяЭЖШыСП | 1 mol CO2ЁЂ3 mol H2 | a mol CO2ЁЂb mol H2ЁЂc mol CH3OH(g)ЁЂc mol H2O(g) |
ШєМзжаЦНКтКѓЦјЬхЕФбЙЧПЮЊПЊЪМЪБЕФ ЃЌвЊЪЙЦНКтКѓввгыМзжаЯрЭЌзщЗжЕФЬхЛ§ЗжЪ§ЯрЕШЃЌЧвЦ№ЪМЪБЮЌГжЗДгІФцЯђНјааЃЌдђcЕФШЁжЕЗЖЮЇЮЊ________ЁЃ
ЃЌвЊЪЙЦНКтКѓввгыМзжаЯрЭЌзщЗжЕФЬхЛ§ЗжЪ§ЯрЕШЃЌЧвЦ№ЪМЪБЮЌГжЗДгІФцЯђНјааЃЌдђcЕФШЁжЕЗЖЮЇЮЊ________ЁЃ
(3)гУ0.10 molЁЄLЃ1бЮЫсЗжБ№ЕЮЖЈ20.00 mL 0.10 molЁЄLЃ1ЕФNaOHШмвККЭ20.00 mL 0.10 molЁЄLЃ1АБЫЎЫљЕУЕФЕЮЖЈЧњЯпШчЯТЃК

ЧыжИГібЮЫсЕЮЖЈАБЫЎЕФЧњЯпЮЊ________(ЬюЁАAЁБЛђЁАBЁБ)ЃЌЧыаДГіЧњЯпaЕуЫљЖдгІЕФШмвКжаИїРызгХЈЖШгЩДѓЕНаЁЕФХХСаЫГађ________ЁЃ