ЬтФПФкШн
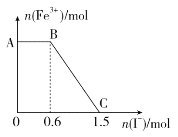
ЁОЬтФПЁПдкКуЮТКуШнЕФУмБеШнЦїжаЃЌЗЂЩњЗДгІЃК3A(g)ЃЋB(g)![]() xC(g)ЁЃ
xC(g)ЁЃ
Ђё.НЋ3molAКЭ2molBдквЛЖЈЬѕМўЯТЗДгІЃЌДяЦНКтЪБCЕФЬхЛ§ЗжЪ§ЮЊaЁЃ
Ђђ.ШєЦ№ЪМЪБAЁЂBЁЂCЭЖШыЕФЮяжЪЕФСПЗжБ№ЮЊn(A)ЁЂn(B)ЁЂn(C)ЃЌЦНКтЪБCЕФЬхЛ§ЗжЪ§вВЮЊaЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
A.ШєЂёДяЦНКтЪБЃЌМгШы3molAЃЌдђжиаТДяЕНЦНКтЪБCЕФЬхЛ§ЗжЪ§вЛЖЈМѕаЁ
B.ШєЯђЂёЦНКтЬхЯЕжадйМгШы3molAКЭ2molBЃЌCЕФЬхЛ§ЗжЪ§ШєДѓгкaЃЌПЩЖЯЖЈx>4
C.ШєxЃН2ЃЌдђЂђЬхЯЕЦ№ЪМЮяжЪЕФСПгІТњзу3n(B)>n(A)ЃЋ3
D.ШєЂђЬхЯЕЦ№ЪМЮяжЪЕФСПТњзу3n(C)ЃЋ8n(A)ЃН12n(B)ЃЌдђПЩХаЖЯxЃН4
ЁОД№АИЁПD
ЁОНтЮіЁП
AЃЎгЩгкxжЕВЛШЗЖЈЃЌдђШєЂёДяЦНКтЪБЃЌМгШы3molAЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌжиаТДяЕНЦНКтЪБЩњГЩЕФCЮоЗЈМЦЫуЃЌCЕФЬхЛ§ЗжЪ§ПЩФмдіДѓвВПЩФмМѕаЁЃЌЙЪAДэЮѓЃЛ
BЃЎвђШнЦїЬхЛ§ВЛБфЃЌШєx=4ЪБЃЌCЕФЬхЛ§ЗжЪ§гІЮЊaЃЌЯжДѓгкaЃЌxгІаЁгк4ЃЌДйНјЦНКте§ЯђвЦЖЏЃЌЙЪBДэЮѓЃЛ
CЃЎгЩКуЮТКуШнЪБЃЌЕБn(A)ЁЂn(B)ЁЂn(C)ЮЊЦ№ЪМЮяжЪЕФСПЃЌЦНКтЪБn(C)ЕФЬхЛ§ЗжЪ§ШдЮЊaЃЌдђn(A)+![]() n(C)=3ЃЌn(B)+
n(C)=3ЃЌn(B)+![]() n(C)=2ЃЌШєx=2ЃЌЖўепСЊЪНПЩЕУ3n(B)=n(A)+3ЃЌЙЪCДэЮѓЃЛ
n(C)=2ЃЌШєx=2ЃЌЖўепСЊЪНПЩЕУ3n(B)=n(A)+3ЃЌЙЪCДэЮѓЃЛ
DЃЎгЩКуЮТКуШнЪБЃЌЕБn(A)ЁЂn(B)ЁЂn(C)ЮЊЦ№ЪМЮяжЪЕФСПЃЌЦНКтЪБn(C)ЕФЬхЛ§ЗжЪ§ШдЮЊaЃЌn(A)+![]() n(C)=3ЃЌn(B)+
n(C)=3ЃЌn(B)+![]() n(C)=2ЃЌШєx=4ЃЌЖўепСЊЪНПЩЕУ3n(C)+8n(A)ЈT12n(B)ЃЌЙЪDе§ШЗЃЛ
n(C)=2ЃЌШєx=4ЃЌЖўепСЊЪНПЩЕУ3n(C)+8n(A)ЈT12n(B)ЃЌЙЪDе§ШЗЃЛ
Д№АИбЁDЁЃ

 ЪюМйзївЕКЃбрГіАцЩчЯЕСаД№АИ
ЪюМйзївЕКЃбрГіАцЩчЯЕСаД№АИ БОЭСНЬИЈгЎдкЪюМйИпаЇМйЦкзмИДЯАдЦФЯПЦММГіАцЩчЯЕСаД№АИ
БОЭСНЬИЈгЎдкЪюМйИпаЇМйЦкзмИДЯАдЦФЯПЦММГіАцЩчЯЕСаД№АИЁОЬтФПЁПМзДМЪЧживЊЕФЛЏЙЄдСЯЃЌРћгУУКЛЏЙЄжаЩњВњЕФCOЁЂCO2КЭH2ПЩжЦШЁМзДМЕШгаЛњЮяЃЌЗЂЩњЕФЗДгІгаЃК
ЂйCO(g)+2H2(g)![]() CH3OH(g) ЁїH1=Ѓ99kJmol-1
CH3OH(g) ЁїH1=Ѓ99kJmol-1
ЂкCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ЁїH2
CH3OH(g)+H2O(g) ЁїH2
ЯрЙиЮяжЪЕФЛЏбЇМќМќФмЪ§ОнШчЯТЃКCH3OHНсЙЙЪН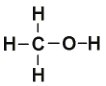
ЛЏбЇМќ | C=O(CO2) | HЁЊH | CЁЊO | HЁЊO | CЁЊH |
E/(kJЁЄmol-1) | 803 | 436 | 343 | 465 | 413 |
(1)ИУЗДгІЁїH2=____________ЁЃ
(2)ЙигкЗДгІЂйЯТСаЫЕЗЈЃЌе§ШЗЕФЪЧ____________ЁЃ
A.ИУЗДгІдкШЮКЮЮТЖШЯТЖМФмздЗЂНјаа
B.Щ§ИпЮТЖШЃЌе§ЗДгІЫйТЪдіДѓЃЌФцЗДгІЫйТЪМѕаЁ
C.ЪЙгУДпЛЏМСЃЌВЛФмЬсИпCOЕФзЊЛЏТЪ
D.діДѓбЙЧПЃЌИУЗДгІЕФЛЏбЇЦНКтГЃЪ§ВЛБф
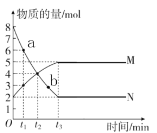
(3)дкФГЮТЖШЯТЃЌНЋ1.0moCOгы2.0molH2ГфШы2LЕФПеИжЦПжаЃЌЗЂЩњЗДгІЂйЃЌдкЕк5minЪБДяЕНЛЏбЇЦНКтзДЬЌЃЌДЫЪБМзДМЕФЮяжЪЕФСПЗжЪ§ЮЊ0.1ЁЃдкЕк10minЁЂ20minЪБЗжБ№ИФБфЗДгІЬѕМўЃЌМзДМЕФХЈЖШдкВЛЭЌЬѕМўЯТЕФБфЛЏзДПіШчЭМЫљЪОЃК

ЂйДгЗДгІПЊЪМЕН5minЪБЃЌЩњГЩМзДМЕФЦНОљЫйТЪЮЊ____________ЁЃ
ЂкH2ЕФЦНКтзЊЛЏТЪІС=____________%ЃЌЛЏбЇЦНКтГЃЪ§K=____________ЁЃ
Ђл1minЪБЃЌІде§____________ІдФц(ЬюЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБ)
Ђм1mimЪБІде§____________4minЪБІдФц(ЬюЁАДѓгкЁБЁАаЁгкЁБЛђЁАЕШгкЁБ)
ЂнБШНЯМзДМдк7ЁЋ8minЁЂ12ЁЋ13minКЭ25ЁЋ27minЪБЦНОљЗДгІЫйТЪ[ЦНОљЗДгІЫйТЪЗжБ№вдІд(7ЁЋ8)ЁЂІд(12ЁЋ13)ЁЂІд(25ЁЋ27)БэЪОЕФДѓаЁ____________ЁЃ
ЂоШєНЋИжЦПЛЛГЩЭЌШнЛ§ЕФОјШШШнЦїЃЌжиИДЩЯЪіЪдбщЃЌЦНКтЪБМзДМЕФЮяжЪЕФСПЗжЪ§____________0.1(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁА=ЁБ)ЁЃ
ЁОЬтФПЁПЯТСаИљОнЪЕбщВйзїКЭЯжЯѓЫљЕУГіЕФНсТле§ШЗЕФЪЧЃЈ ЃЉ
ЪЕбщВйзї | ЪЕбщЯжЯѓ | НсТл | |
A | ЯђФГШмвКжаМгШыбЮЫсЫсЛЏЕФBaCl2ШмвК | ГіЯжАзЩЋГСЕэ | ШмвКжавЛЖЈКЌгаSO |
B | гУВЌЫПеКШЁД§ВтвКЃЌдкОЦОЋЕЦЛ№бцЩЯзЦЩе | бцЩЋЮЊЛЦЩЋ | Д§ВтвКжаПЯЖЈВЛКЌK+ |
C | ЕэЗлгыЯЁСђЫсЕФЛьКЯвКМгШШКѓЃЌдйМгШыаТжЦCu(OH)2ЃЌМгШШ | ЮоУїЯдЯжЯѓ | ВЛФмШЗЖЈЕэЗлУЛгаЗЂЩњЫЎНт |
D | НЋЪЏРЏгЭМгЧПШШВњЩњЕФЦјЬхЭЈШыфхЕФЫФТШЛЏЬМШмвКжа | ШмвКЭЪЩЋ | ВњЮяВЛЖМЪЧЭщЬў |
A.AB.BC.CD.D