��Ŀ����
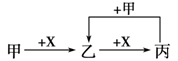
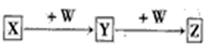
��֪A��B��C��D��E��X������ͼʾת����ϵ(����������ͷ�Ӧ������)��
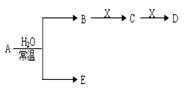
��1����EΪ����A��ˮ��Ӧ�Ļ�ѧ����ʽ ����ʾX��Һ�ʼ��Ե����ӷ���ʽΪ ���ýṹʽ��ʾC���ӣ� ��
�ڵ�XΪ��������ʱ����X��B��ϡ��Һ��Ӧ����C�����ӷ�Ӧ����ʽΪ ��
��2����EΪ�����������壬DΪ��ɫ��״������A�Ļ�ѧʽ������ ��B�к��еĻ�ѧ������Ϊ ��C��X��Ӧ�����ӷ���ʽΪ ��
��3����A��B��Ϊ���嵥�ʣ�D����ˮ������һ�������·������淴Ӧ����C��һ�ֿ�ȼ�����嵥�ʣ���ÿ��淴Ӧ�Ļ�ѧ����ʽΪ ��t��ʱ�����ܱպ��ݵ�ij������Ͷ������ʵ�����D��ˮ������һ��ʱ���ﵽƽ�⣬���¶��·�Ӧ�Ļ�ѧƽ�ⳣ��K=1����D��ת����Ϊ ��

��1����EΪ����A��ˮ��Ӧ�Ļ�ѧ����ʽ ����ʾX��Һ�ʼ��Ե����ӷ���ʽΪ ���ýṹʽ��ʾC���ӣ� ��
�ڵ�XΪ��������ʱ����X��B��ϡ��Һ��Ӧ����C�����ӷ�Ӧ����ʽΪ ��
��2����EΪ�����������壬DΪ��ɫ��״������A�Ļ�ѧʽ������ ��B�к��еĻ�ѧ������Ϊ ��C��X��Ӧ�����ӷ���ʽΪ ��
��3����A��B��Ϊ���嵥�ʣ�D����ˮ������һ�������·������淴Ӧ����C��һ�ֿ�ȼ�����嵥�ʣ���ÿ��淴Ӧ�Ļ�ѧ����ʽΪ ��t��ʱ�����ܱպ��ݵ�ij������Ͷ������ʵ�����D��ˮ������һ��ʱ���ﵽƽ�⣬���¶��·�Ӧ�Ļ�ѧƽ�ⳣ��K=1����D��ת����Ϊ ��
��1��3NO2 +H2O=2HNO3 + NO (2��)
��CO32-+H2O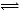 HCO3-+OH-(2��) O="C=O" (1��)
HCO3-+OH-(2��) O="C=O" (1��)
��Fe + 4H+ +NO3-= Fe3+ + NO��+2H2O (2��)
��2��Na (��Na2O2) (1��)�� ���Ӽ��ͼ��Թ��ۼ�(1��)��Al3+��3AlO2-��6H2O=4Al(OH)3��(2��)
��3��CO(g)+H2O(g)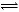 CO2(g)+H2(g) (2��) 50% (1��)
CO2(g)+H2(g) (2��) 50% (1��)
��CO32-+H2O
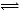 HCO3-+OH-(2��) O="C=O" (1��)
HCO3-+OH-(2��) O="C=O" (1��)��Fe + 4H+ +NO3-= Fe3+ + NO��+2H2O (2��)
��2��Na (��Na2O2) (1��)�� ���Ӽ��ͼ��Թ��ۼ�(1��)��Al3+��3AlO2-��6H2O=4Al(OH)3��(2��)
��3��CO(g)+H2O(g)
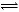 CO2(g)+H2(g) (2��) 50% (1��)
CO2(g)+H2(g) (2��) 50% (1��)�����������1��EΪ�ǽ��������Aһ�����ǽ���Ԫ�أ�������ˮ��Ӧ����NO2��������֤��X��Fe���������ٵ�X�Ǽ�����Һ��C����һ�������������У���22�����ӣ�ΪCO2��X��̼���Σ�CO32-+H2O
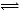 HCO3-+OH-�Լ��ԡ��ڵ�XΪ�������ʣ������Feʱ����Fe��HNO3��ϡ��Һ��Ӧ����NO��Fe + 4H+ +NO3-= Fe3+ + NO��+2H2O��
HCO3-+OH-�Լ��ԡ��ڵ�XΪ�������ʣ������Feʱ����Fe��HNO3��ϡ��Һ��Ӧ����NO��Fe + 4H+ +NO3-= Fe3+ + NO��+2H2O����2��DΪ��ɫ��״���������뵽Al(OH)3��OH-�����ڼ�����Һ����EΪ�����������壬��A��Na��Na2O2��B��NaOH��XΪAl3+��C��AlO2-��C��X��ӦAl3+��3AlO2-��6H2O=4Al(OH)3����
��3��D����ˮ������һ�������·������淴Ӧ����C��һ�ֿ�ȼ�����嵥��(ˮ���ɵ�һ����H2��O2�е�һ�֣�ѡ��H2)��D��CO��C��CO2����ӦΪCO(g)+H2O(g)
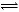 CO2(g)+H2(g)����Ӧ��������Ϊ1��K=
CO2(g)+H2(g)����Ӧ��������Ϊ1��K=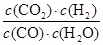 =1��˵��ƽ��ʱc(CO)=c(CO2)��CO��ת����Ϊ050%��
=1��˵��ƽ��ʱc(CO)=c(CO2)��CO��ת����Ϊ050%��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ������˵������ȷ����
������˵������ȷ����