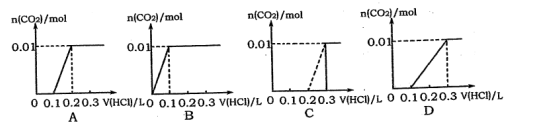
��Ŀ����
��ѧ��ѧ����I�̲��У��漳�˸��������������ϵ����Ҫ�������ǽ������仯�����ش�
��1��д������ԭ�ӽṹʾ��ͼ______��
��2��д����������ˮ��Ӧ�����ӷ���ʽ______����������Һ�м�����ȥ����Ĥ����Ƭ����д����ѧ��Ӧ����ʽ______��
��3����һ�෴ӦΪ������+��������µ���+���������д���������෴Ӧ���ҷ�������Ҫ��Ļ�ѧ��Ӧ����ʽ��
�����û�������______��
�����û����ǽ���______��
�ǽ���ת�����ǽ���______��
��1��д������ԭ�ӽṹʾ��ͼ______��
��2��д����������ˮ��Ӧ�����ӷ���ʽ______����������Һ�м�����ȥ����Ĥ����Ƭ����д����ѧ��Ӧ����ʽ______��
��3����һ�෴ӦΪ������+��������µ���+���������д���������෴Ӧ���ҷ�������Ҫ��Ļ�ѧ��Ӧ����ʽ��
�����û�������______��
�����û����ǽ���______��
�ǽ���ת�����ǽ���______��
��1������13��Ԫ�أ���ԭ�Ӻ�����13�����ӣ�����ԭ�ӽṹʾ��ͼΪ��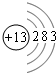 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2���ƺ�ˮ��Ӧ�����������ƺ����������ӷ�Ӧ����ʽΪ��2Na+2H2O=2Na++2OH-+H2������������������Һ��Ӧ����ƫ�����ƺ���������Ӧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����2Al+2NaOH+2H2O=2NaAlO2+3H2���� ��3�������ȷ�Ӧ���ڷ�����������Ӧ����ʽΪ��2Al+Fe2O3
Al2O3+2Fe��
��þ�Ͷ�����̼�ķ�Ӧ������������Ӧ����ʽΪ��2Mg+CO2
2MgO+C��
��̼�Ͷ�������ķ�Ӧ������������Ӧ����ʽΪ��C+SiO2
Si+2CO
�ʴ�Ϊ��2Al+Fe2O3
Al2O3+2Fe��2Mg+CO2
2MgO+C��
C+SiO2
Si+2CO��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2���ƺ�ˮ��Ӧ�����������ƺ����������ӷ�Ӧ����ʽΪ��2Na+2H2O=2Na++2OH-+H2������������������Һ��Ӧ����ƫ�����ƺ���������Ӧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Na+2H2O=2Na++2OH-+H2����2Al+2NaOH+2H2O=2NaAlO2+3H2���� ��3�������ȷ�Ӧ���ڷ�����������Ӧ����ʽΪ��2Al+Fe2O3
| ||
��þ�Ͷ�����̼�ķ�Ӧ������������Ӧ����ʽΪ��2Mg+CO2
| ||
��̼�Ͷ�������ķ�Ӧ������������Ӧ����ʽΪ��C+SiO2
| ||
�ʴ�Ϊ��2Al+Fe2O3
| ||
| ||
C+SiO2
| ||

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ