��Ŀ����
̼��������ҹ���Ҫ�ĵ���Ʒ��֮һ���������������������ӷ���ʧ��Ϊ�˼�����������ȷ�����ʩ����������ⶨ�京������
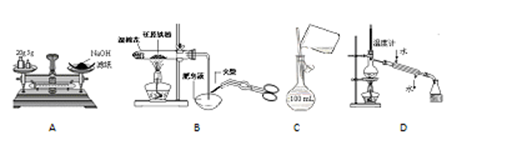
��ijѧ�������һ���Բⶨ������̼������Ӳⶨ�������ķ���������Ʒ����Բ����ƿ�У�


��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ ��
��2����Һ©���е�Һ�����ʺϵ��� ��
��3����������װ�������� ��
����������гɷ��ǣ�NH4��2SO4��������ü�ȩ���ⶨ����������ȩ���ǻ��ڼ�ȩ��һ������������ã������൱�����ᣬ��ӦΪ2��NH4��2SO4+6HCHO����CH2��6N4 +2H2SO4 + 6H2O,���ɵ��������������Ʊ���Һ�ζ����Ӷ��ⶨ���ĺ������������£�
��1���ò�������ȡ���壨NH4��2SO4��Ʒ0��6g���ձ��У�����Լ30mL����ˮ�ܽ⣬�������100mL��Һ���� �����ʽ����ʽ�����ζ���ȷȡ��20��00mL����Һ����ƿ�У�����18%���Լ�ȩ��Һ5mL������5min����1~2�� ָʾ������֪�ζ��յ��pHԼΪ8��8������Ũ��Ϊ0��08mol/L�������Ʊ���Һ�ζ����������±���
��ζ��յ�ʱ������Ϊ ���ɴ˿ɼ��������Ʒ�еĵ�����������Ϊ ��
��2���ڵζ�ʵ��������ֵζ��õļ�ʽ�ζ��ܲ��������ڳ��������ݣ��ζ���ʼʱ�����ݣ����ʵ��ⶨ�ĺ�������ʵ��ֵ ���ƫ��ƫС������Ӱ�족����
������ⶨ̼������еĺ�����ʱ��ʹ�ü�ȩ���Ƿ���� ����ǡ����������� ��
��ijѧ�������һ���Բⶨ������̼������Ӳⶨ�������ķ���������Ʒ����Բ����ƿ�У�


��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ ��
��2����Һ©���е�Һ�����ʺϵ��� ��
| A��ϡ���� | B��ϡ���� | C��Ũ���� | D���������� |
����������гɷ��ǣ�NH4��2SO4��������ü�ȩ���ⶨ����������ȩ���ǻ��ڼ�ȩ��һ������������ã������൱�����ᣬ��ӦΪ2��NH4��2SO4+6HCHO����CH2��6N4 +2H2SO4 + 6H2O,���ɵ��������������Ʊ���Һ�ζ����Ӷ��ⶨ���ĺ������������£�
��1���ò�������ȡ���壨NH4��2SO4��Ʒ0��6g���ձ��У�����Լ30mL����ˮ�ܽ⣬�������100mL��Һ���� �����ʽ����ʽ�����ζ���ȷȡ��20��00mL����Һ����ƿ�У�����18%���Լ�ȩ��Һ5mL������5min����1~2�� ָʾ������֪�ζ��յ��pHԼΪ8��8������Ũ��Ϊ0��08mol/L�������Ʊ���Һ�ζ����������±���
| �ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 1��20 | 16��21 |
| 2 | 3��00 | 18��90 |
| 3 | 4��50 | 19��49 |
��ζ��յ�ʱ������Ϊ ���ɴ˿ɼ��������Ʒ�еĵ�����������Ϊ ��
��2���ڵζ�ʵ��������ֵζ��õļ�ʽ�ζ��ܲ��������ڳ��������ݣ��ζ���ʼʱ�����ݣ����ʵ��ⶨ�ĺ�������ʵ��ֵ ���ƫ��ƫС������Ӱ�족����
������ⶨ̼������еĺ�����ʱ��ʹ�ü�ȩ���Ƿ���� ����ǡ����������� ��
��1��b-e,f-h,g-c (2)B (3)��ֹ������ˮ�Ͷ�����̼����װ��Ӱ��ʵ������
��1����ʽ ��̪ ��Һ����ɫ��Ϊdz��ɫ ��30s�ڲ���ɫ 14% ��2��ƫС
�����Ϊ���������Ʊ���Һ�ζ�ʱ����Һ��HCO3���е�H+Ҳ������������к�
��1����ʽ ��̪ ��Һ����ɫ��Ϊdz��ɫ ��30s�ڲ���ɫ 14% ��2��ƫС
�����Ϊ���������Ʊ���Һ�ζ�ʱ����Һ��HCO3���е�H+Ҳ������������к�
�����������1�����⿼��ʵ��װ�õ�ѡ������ӣ�ʵ��װ�õ�����˳��Ϊ����װ�á�����װ�á�����װ�á����ʻ��ռ�װ�á�β������װ�á�һ�������ǰ�������ں�����ȼ�չܳ��ӣ��������ǰ������ϴ��ƿ�������̳��������ø���ܣ����С��������������֪��װ������������Һ��ϴ��ƿΪ������̼������װ�ã�Ϊ��֤ʵ����ȷ�������ֹˮ�������������ĸ��ţ�Ϊ��֤��Һ©����Һ��˳�����£�Ӧѡ�ڶ���װ����Ϊ������̼�ķ���װ�ã����������������Ӵ���Ϊ��b-e,f-h,g-c����2������������ӷ�������Ŷ�����̼�IJⶨ������������Һ����Ʒ��Ӧ�������ɶ�����̼�����Է�Һ©���ڵ�Һ��ӦΪϡ���ᣬѡB����3����������װ��������Ϊ��ֹ������ˮ�Ͷ�����̼����װ��Ӱ��ʵ������
��1����NH4��2SO4����ǿ�������Σ�ˮ�������ԣ���������ʽ�ζ���ȷȡ��20��00mL����Һ���ζ��յ��pHԼΪ8��8����̪��ɫ��ΧΪ8.2����10��Ӧ�÷�̪��ָʾ������Ũ��Ϊ0��08mol/L�������Ʊ���Һ�ζ�����ζ��յ�ʱ������Ϊ��Һ����ɫ��Ϊdz��ɫ ����30s�ڲ���ɫ�������������֪���ڶ����������ϴ�Ӧ��������������������Һ�����Ϊ15.00mL ���������Ӧ�õ�ԭ������������֮��Ĺ�ϵʽ��N����NaOH,�������ݼ���ɵã�0.6g��Ʒ�к���ԭ�ӵ����ʵ���Ϊ0��08mol/L��0.015L��5=0.006mol��������Ϊ0.084g,��������Ϊ14%����2���ڵζ�ʵ��������ֵζ��õļ�ʽ�ζ��ܲ��������ڳ��������ݣ��ζ���ʼʱ�����ݣ�������������������Һ�����ƫС�����ʵ��ⶨ�ĺ�������ʵ��ֵƫС��
������ⶨ̼������еĺ�����ʱ��ʹ�ü�ȩ������������������Ϊ���������Ʊ���Һ�ζ�ʱ����Һ��HCO3���е�H+Ҳ������������к͡�

��ϰ��ϵ�д�
�����Ŀ