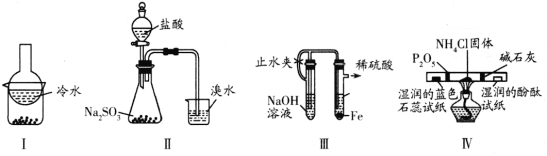
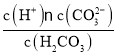ЬтФПФкШн
ЁОЬтФПЁПгаЛњЮяAГЃгУгкЪГЦЗаавЕЃЌвбжЊ9.2 g AдкзуСПЕФO2жаГфЗжШМЩеЃЌНЋЩњГЩЕФЛьКЯЦјЬхвРДЮЭЈЙ§зуСПЕФХЈСђЫсКЭМюЪЏЛвЃЌЖўепЗжБ№діжи3.6gКЭ8.8 gЃЌОМьбщЪЃгрЦјЬхЮЊO2ЁЃ
(1)гаЛњЮяAЕФжЪЦзЭМШчЯТЭМЫљЪОЃЌДгЭМжаПЩжЊЦфЯрЖдЗжзгжЪСПЪЧ___ЁЃ
(2)AЕФНсЙЙМђЪНЮЊ___ЁЃ
(3)гУAНјааЯТСаЗДгІ
ЂйвјОЕЗДгІЃКAдкНјаавјОЕЗДгІжЎЧАБиаыдкЦфжаМгШывЛЖЈСПЕФ_______ЃЌвђЮЊ_______ЁЃ
ЂкAЗЂЩњвјОЕЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________ЁЃ
ЂлаДГіAКЭввДМЗДгІЕФЛЏбЇЗНГЬЪН___________ЁЃ
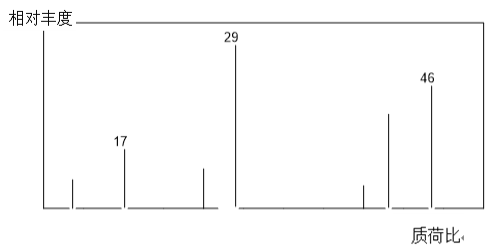
ЁОД№АИЁП46 HCOOH ЧтбѕЛЏФЦШмвК ЗДгІБиаыдкМюадЕФЬѕМўЯТЗЂЩњ ![]()
![]()
![]()
ЁОНтЮіЁП
(1)гаЛњЮяAЕФжЪЦзЭМжазюДѓжЪКЩБШЮЊ46ЃЛ
(2) ХЈСђЫсдіжи3.6gЮЊЫЎЕФжЪСПЃЌМюЪЏЛвдіжи8.8gЮЊЖўбѕЛЏЬМЕФжЪСПЃЌИљОндзгЪиКуШЗЖЈCЁЂHдзгЪ§ФПЃЌНсКЯЯрЖдЗжзгжЪСПШЗЖЈбѕдзгЪ§ФПЃЌНјЖјШЗЖЈгаЛњЮяЗжзгЪНЃЛ
(3) МзЫсКЌгаШЉЛљЃЌПЩвдЗЂЩњвјОЕЗДгІЃЌвјОЕЗДгІЕФЬѕМўЪЧдкМюадЬѕМўЯТЗЂЩњЃЛМзЫсКЭввДМПЩвдЗЂЩњѕЅЛЏЗДгІЁЃ
(1) гаЛњЮяAЕФжЪЦзЭМжазюДѓжЪКЩБШЮЊ46ЃЌдђЦфЯрЖдЗжзгжЪСПЪЧ46ЃЛ
(2) 9.2 g AЕФЮяжЪЕФСПЮЊ![]() =0.2molЃЌ3.6gЫЎЕФЮяжЪЕФСПЮЊ
=0.2molЃЌ3.6gЫЎЕФЮяжЪЕФСПЮЊ![]() =0.2molЃЌn(H)=0.4molЃЌ8.8gЖўбѕЛЏЬМЕФЮяжЪЕФСПЮЊЃК
=0.2molЃЌn(H)=0.4molЃЌ8.8gЖўбѕЛЏЬМЕФЮяжЪЕФСПЮЊЃК![]() =0.2molЃЌn(C)=n(CO2)=0.2molЃЌAЗжзгжаN(C)=
=0.2molЃЌn(C)=n(CO2)=0.2molЃЌAЗжзгжаN(C)=![]() =1ЃЌN(H)=
=1ЃЌN(H)=![]() =2ЃЌN(O)=
=2ЃЌN(O)=![]() =2ЃЌЙЪгаЛњЮяAЗжзгЪНЮЊCH2O2ЃЌЦфНсЙЙМђЪНЮЊHCOOHЃЛ
=2ЃЌЙЪгаЛњЮяAЗжзгЪНЮЊCH2O2ЃЌЦфНсЙЙМђЪНЮЊHCOOHЃЛ
(3) ЂйвјОЕЗДгІЕФЬѕМўЪЧдкМюадЬѕМўЯТЗЂЩњЃЌдкМзЫсНјаавјОЕЗДгІЧАЃЌБиаыдкЦфжаМгШывЛЖЈСПЕФМюЃЌПЩвдМгNaOHШмвКЃЛ
ЂкМзЫсКЌгаШЉЛљЃЌФмЗЂЩњвјОЕЗДгІЃЌЗДгІЗНГЬЪНЮЊЃК![]() ЃЛ
ЃЛ
ЂлHCOOHКЭввДМЗЂЩњѕЅЛЏЗДгІЕФЛЏбЇЗНГЬЪНЮЊ![]() ЁЃ
ЁЃ

ЁОЬтФПЁПввЫсвьЮьѕЅЪЧзщГЩУлЗфаХЯЂЫиЕФГЩЗжжЎвЛ,ОпгаЯуНЖЕФЯуЮЖ,ЪЕбщЪвжЦБИввЫсвьЮьѕЅЕФЗДгІЁЂзАжУЪОвтЭМКЭгаЙиЪ§ОнШчЯТ:
![]() +
+![]()
![]()
![]() +H2O
+H2O
ЯрЖдЗжзгжЪСП | УмЖШ/(gЁЄcm-3) | ЗаЕу/Ёц | ЫЎжаШмНтад | |
вьЮьДМ | 88 | 0.812 3 | 131 | ЮЂШм |
ввЫс | 60 | 1.049 2 | 118 | Шм |
ввЫсвьЮьѕЅ | 130 | 0.867 0 | 142 | ФбШм |
ЪЕбщВНжш:
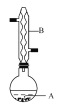
дкAжаМгШы4.4 gвьЮьДМЁЂ6.0 gввЫсЁЂЪ§ЕЮХЈСђЫсКЭ2ЁЋ3ЦЌЫщДЩЦЌ,ПЊЪМЛКТ§МгШШA,ЛиСї50 min,ЗДгІвКРфжСЪвЮТКѓЕЙШыЗжвКТЉЖЗжа,ЗжБ№гУЩйСПЫЎЁЂБЅКЭЬМЫсЧтФЦШмвК КЭЫЎ ЯДЕг,ЗжГіЕФВњЮяМгШыЩйСПЮоЫЎMgSO4ЙЬЬх,ОВжУЦЌПЬ,Й§ТЫГ§ШЅMgSO4ЙЬЬх,НјааеєСѓДПЛЏ,ЪеМЏ140ЁЋ143ЁцСѓЗж,ЕУввЫсвьЮьѕЅ3.9 gЁЃЛиД№ЯТСаЮЪЬт:
(1)вЧЦїBЕФУћГЦЪЧ________ЃЌЫќЕФзїгУЪЧ____________________ЁЃ
(2)дкЯДЕгВйзїжа,ЕквЛДЮЫЎЯДЕФжївЊФПЪЧ___________________________
ЕкЖўДЮЫЎЯДЕФжївЊФПЕФЪЧ_______________________________________ЁЃ
(3)дкЯДЕгЁЂЗжвКВйзїжа,гІГфЗжеёЕД,ШЛКѓОВжУ,Д§ЗжВуКѓ____(ЬюБъКХ)ЁЃ
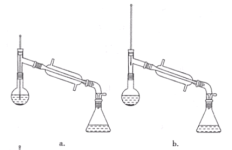
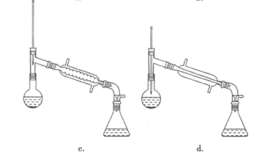
a.жБНгНЋввЫсвьЮьѕЅДгЗжвКТЉЖЗЕФЩЯПкЕЙГі
b.жБНгНЋввЫсвьЮьѕЅДгЗжвКТЉЖЗЕФЯТПкЗХГі
c.ЯШНЋЫЎВуДгЗжвКТЉЖЗЕФЯТПкЗХГі,дйНЋввЫсвьЮьѕЅДгЯТПкЗХГі
d.ЯШНЋЫЎВуДгЗжвКТЉЖЗЕФЯТПкЗХГі,дйНЋввЫсвьЮьѕЅДгЩЯПкЕЙГі
(4)БОЪЕбщжаМгШыЙ§СПввЫсЕФФПЕФЪЧ_____________________________ЁЃ
(5)ЪЕбщжаМгШыЩйСПЮоЫЎMgSO4ЕФФПЕФЪЧ___________________________ЁЃ
(6)дкеєСѓВйзїжа,вЧЦїбЁдёМААВзАЖМе§ШЗЕФЪЧ____(ЬюБъКХ)ЁЃ
(7)БОЪЕбщЕФВњТЪЪЧ____(ЬюБъКХ)ЁЃ
a.30% b.40% c. 60% d.90%
(8)дкНјааеєСѓВйзїЪБ,ШєДг130ЁцБуПЊЪМЪеМЏСѓЗж,ЛсЪЙЪЕбщЕФВњТЪЦЋ____(ЬюЁАИпЁБЛђЁАЕЭЁБ),ЦфдвђЪЧ____________________________________ЁЃ
ЁОЬтФПЁПЙЄвЕЩЯвдИѕЬњПѓ(жївЊГЩЗжЪЧFeOЁЄCr2O3ЃЌКЌЩйСПMgCO3ЁЂAl2O3ЁЂSiO2ЕШ)ЮЊдСЯжЦШЁИѕЫсФЦ(Na2CrO4)ОЇЬхЃЌЦфЙЄвеСїГЬШчЯТЃК

вбжЊЃКЂйЃЋ3МлCrдкЫсадШмвКжааджЪЮШЖЈЃЌЕБpH>9ЪБвдCrO![]() аЮЪНДцдкЧввзБЛбѕЛЏЁЃ
аЮЪНДцдкЧввзБЛбѕЛЏЁЃ
ЂкГЃЮТЯТЃЌВПЗжбєРызгвдЧтбѕЛЏЮяаЮЪНГСЕэЪБШмвКЕФpHШчЯТЃК
бєРызг | Fe3ЃЋ | Fe2ЃЋ | Mg2ЃЋ | Al3ЃЋ | Cr3ЃЋ |
ПЊЪМГСЕэЪБЕФpH | 1ЃЎ9 | 7ЃЎ0 | 9ЃЎ1 | ЁЊ | ЁЊ |
ГСЕэЭъШЋЪБЕФpH | 3ЃЎ2 | 9ЃЎ0 | 11ЃЎ1 | 4ЃЎ7(>10ШмНт) | 5ЃЎ6(>9ШмНт) |
(1)ЬсИпЫсНўЫйТЪЕФДыЪЉга__________________________________(Д№СНЬѕ)ЁЃ
(2)ТЫдќ1ЕФжївЊГЩЗжЪЧ_____ЃЛТЫдќ2ЕФжївЊГЩЗжЪЧ_____ЃЛТЫдќ3ЕФжївЊГЩЗжЪЧ_____ЁЃ
(3)СїГЬжаСНДЮЪЙгУСЫH2O2НјаабѕЛЏЃЌH2O2ЕФЕчзгЪНЮЊ___________ЃЛЕквЛДЮбѕЛЏЪБЗДгІЕФРызгЗНГЬЪНЮЊ_____________________ЃЛЕкЖўДЮбѕЛЏЪБФПЕФЪЧ____________________ЁЃ
(4)СїГЬЭМжаЁА ЁБФкЕФВйзїЪЧ________________ЁЂЯДЕгЁЂИЩдяЁЃ
(5)Г§ШЅЫсадЗЯЫЎжаКЌгаЕФCr2O72-ПЩвдЪЙгУFeSO4ЃЌВтЕУЗДгІКѓЕФШмвКжаКЌCr3ЃЋЁЂFe2ЃЋЁЂFe3ЃЋЁЂHЃЋЕШбєРызгЁЃИУЗДгІЕФРызгЗНГЬЪНЮЊ_________________________________ЁЃ