��Ŀ����
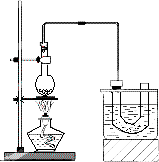
 ��ͼ��ʵ�������������װ��ʾ��ͼ�����Թܢ������μ���2ml����ˮ��4mLŨ���ᡢ4mL��95%���Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ�Ӧ������
��ͼ��ʵ�������������װ��ʾ��ͼ�����Թܢ������μ���2ml����ˮ��4mLŨ���ᡢ4mL��95%���Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ�Ӧ�������ش��������⣺
��1���Թܢ���������������ˮ��ʵ��ʱ���Թܢ��п��ܹ۲쵽���쳣������
��2�����Թܼ����ӵ�б��ͨ���ϳ�����Ŀ����
��3���Թܢ��з�����Ӧ�Ļ�ѧ����ʽ��
��4���÷�Ӧ�����¶���Ϊ��Ҫ�����¶ȹ��ߣ����ʴ���Ƚ��ͣ���ԭ����˷�������Ӧ�⣬����һ����Ҫ��ԭ����
��5�����ôֲ�Ʒ����ϴ�ӣ�
��������1������������ˮ��Ũ�������ǿ�����ԣ���ʵ��ʱ���Թܢ��п��ܹ۲쵽�к���ɫ�������ɣ�
��2���ϳ���б���������ͻ��������ã�
��3����ȡ������ķ���ʽ��C2H5OH+NaBr+H2SO4
C2H5Br+NaHSO4+H2O��
��4���Ҵ����ӷ����¶ȹ��ߣ����²��ʴ���Ƚ��ͣ�
��5�����ôֲ�Ʒ����ϴ�ӣ���Һ�������ܵõ���Ʒ��
��2���ϳ���б���������ͻ��������ã�
��3����ȡ������ķ���ʽ��C2H5OH+NaBr+H2SO4
| �� |
��4���Ҵ����ӷ����¶ȹ��ߣ����²��ʴ���Ƚ��ͣ�
��5�����ôֲ�Ʒ����ϴ�ӣ���Һ�������ܵõ���Ʒ��
����⣺��1����������ˮ�ܹ�ϡ��Ũ���ᣬ��������ˮ���廯�ƻᱻ�����ɺ���ɫ���壬���ֺ���ɫ���壬�ʴ�Ϊ�����ֺ���ɫ���壻
��2�����Թܼ����ӵ�б��ͨ���ϳ�����Ŀ�����������ͻ������ã��ʴ�Ϊ�������ͻ�����
��3����Ӧ�Ļ�ѧ����ʽ�ǣ�C2H5OH+NaBr+H2SO4
C2H5Br+NaHSO4+H2O���ʴ�Ϊ��C2H5OH+NaBr+H2SO4
C2H5Br+NaHSO4+H2O��
��4���¶ȹ��ߣ��ƾ���ӷ������²��ʴ���Ƚ��ͣ��ʴ�Ϊ���ƾ��ӷ���
��5�����ôֲ�Ʒ����ϴ�ӣ���Һ�������ܵõ���Ʒ���ʴ��ǣ���Һ������
��2�����Թܼ����ӵ�б��ͨ���ϳ�����Ŀ�����������ͻ������ã��ʴ�Ϊ�������ͻ�����
��3����Ӧ�Ļ�ѧ����ʽ�ǣ�C2H5OH+NaBr+H2SO4
| �� |
| �� |
��4���¶ȹ��ߣ��ƾ���ӷ������²��ʴ���Ƚ��ͣ��ʴ�Ϊ���ƾ��ӷ���
��5�����ôֲ�Ʒ����ϴ�ӣ���Һ�������ܵõ���Ʒ���ʴ��ǣ���Һ������
���������⿼�����������ȡ��ע�ػ���֪ʶ�Ŀ��飬�ѶȲ��ߣ�

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
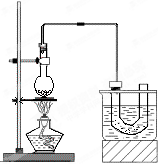 ��ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩
��ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩 ������ʵ���������ȷ����
������ʵ���������ȷ���� ��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ���÷�Ӧʵ��ͨ�������廯����һ��Ũ�ȵ�������Ҵ���Ӧ��ij����С������ʵ�����Ʊ��������װ����ͼ��ʵ������� �����£�
��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ���÷�Ӧʵ��ͨ�������廯����һ��Ũ�ȵ�������Ҵ���Ӧ��ij����С������ʵ�����Ʊ��������װ����ͼ��ʵ������� �����£�