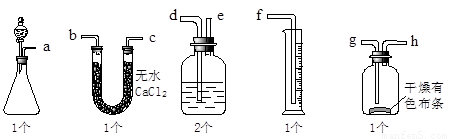��Ŀ����
��10�֣�ÿ��2�֣��ס�������ʵ��С������KMnO4������Һ��H2C2O4��Һ��Ӧ�о�Ӱ�췴Ӧ���ʵ����ء�
��1���÷�Ӧ�����ӷ���ʽΪ����ʾ��H2C2O4��һ������ƽ�ⳣ��Ϊ5.4��10-2��
��
���ʵ�鷽�����£�ʵ��������KMnO4��Һ���Ѽ���H2SO4����
��2�����飺ͨ���ⶨ��λʱ��������CO2��������Ĵ�С���Ƚϻ�ѧ��Ӧ���ʵĴ�С��ʵ��װ����ͼ��ʵ��ʱ��Һ©����A��Һһ���Է��£�A��B�ijɷּ��±���
| ��� | A��Һ | B��Һ |
| �� | 2 mL 0.1 mol/L H2C2O4��Һ | 4 mL 0.01 mol/L KMnO4��Һ |
| �� | 2 mL 0.2 mol/L H2C2O4��Һ | 4 mL 0.01 mol/L KMnO4��Һ |
| �� | 2 mL 0.2 mol/L H2C2O4��Һ | 4 mL 0.01 mol/L KMnO4��Һ������MnSO4 |
��ʵ��̽������ �Ի�ѧ��Ӧ���ʵ�Ӱ�졣�ڷ�Ӧֹ֮ͣǰ����ͬʱ�������������CO2������ɴ�С��˳���� (��ʵ��������)��
��3�����飺ͨ���ⶨKMnO4��Һ��ɫ����ʱ��Ķ������Ƚϻ�ѧ��Ӧ���ʵĴ�С��
ȡ��֧�Թܸ�����2 mL 0.1 mol/L H2C2O4��Һ����ȡ��֧�Թܸ�����4 mL 0.1 mol/L KMnO4��Һ������֧�Թֳܷ����飨����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թܣ���һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ��ϲ�����¼��Һ��ɫ����ʱ�䡣��ʵ��Ŀ�����о� �Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ���� ��
����:��

��10�֣�ÿ��2�֣������������ʵ���Ҫ���������ʵĽṹ����ش��������⣺
(1)��֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ������±���ʾ��
|
������/kJ��mol��1 |
I1 |
I2 |
I3 |
I4 |
|
A |
578 |
1 817 |
2 745 |
11 578 |
|
B |
738 |
1 451 |
7 733 |
10 540 |
Aͨ����____�ۣ�A�ĵ縺��__ __B�ĵ縺��(�����������������)��
(2)��֪������Ϊ300 nm�������Ĺ��������е�����ԼΪ399 kJ��mol��1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ��
��
|
���ۼ� |
C��C |
C��N |
C��S |
|
����/kJ��mol��1 |
347 |
305 |
259 |
(3)�о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���________________��
(4)ij�����ķ��ӽṹ��ͼ��ʾ��������ڲ�����__________(����ĸ)��
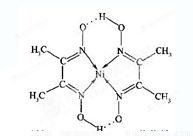
A�����Ӽ� B�����ۼ�
C�������� D����� E�����
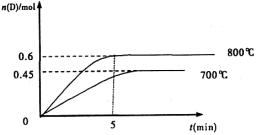
 C��g��+D��g�����ڲ�ͬ�¶��£�D�����ʵ���n��D����ʱ��t�Ĺ�ϵ��ͼ���Իش��������⣺
C��g��+D��g�����ڲ�ͬ�¶��£�D�����ʵ���n��D����ʱ��t�Ĺ�ϵ��ͼ���Իش��������⣺