��Ŀ����
 ��1������ͼ��ʾA��B��C��������������д����������������A
��1������ͼ��ʾA��B��C��������������д����������������A������ƿ
������ƿ
B��Һ©��
��Һ©��
C��ƿ
��ƿ
����2��ijͬѧ��������ƽ�����ձ�����2������ƽƽ����״̬����ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ��һ��������
��������
��������
���ձ���ʵ������27.4
27.4
g��
��3��ָ������3��ʵ���и����ڵ�һ������

A
��ͷ�ιܲ��������Թ�
��ͷ�ιܲ��������Թ�
��B
�Թܿ�Ӧ��������б
�Թܿ�Ӧ��������б
��C
ϴ��װ�õ���Ӧ�����̳�
ϴ��װ�õ���Ӧ�����̳�
����4��Fe��OH��3�����Ʊ��IJ�����
�ڷ�ˮ����μ���5-6�α���FeCl3��aq�������������������Һ�ʺ��ɫ��ֹͣ����
�ڷ�ˮ����μ���5-6�α���FeCl3��aq�������������������Һ�ʺ��ɫ��ֹͣ����
����������1�����ݳ�����������״���⣻
��2����ƽ��������ʱ��ѭ���������ԭ����ƽƽ��ԭ����������������=������������+����������
��3��A����ͷ�ι������Թ��л���Ⱦ�Լ���
B���Թܿ�Ӧ��������б��
C��ϴ��װ�õ���Ӧ�����̳���
��4���ڷ�ˮ����μ���5-6�α���FeCl3��aq�������������������Һ�ʺ��ɫ��ֹͣ���ȣ�
��2����ƽ��������ʱ��ѭ���������ԭ����ƽƽ��ԭ����������������=������������+����������
��3��A����ͷ�ι������Թ��л���Ⱦ�Լ���
B���Թܿ�Ӧ��������б��
C��ϴ��װ�õ���Ӧ�����̳���
��4���ڷ�ˮ����μ���5-6�α���FeCl3��aq�������������������Һ�ʺ��ɫ��ֹͣ���ȣ�
����⣺��1��ͼ�е�ʵ�����������ǣ�������ƿ����Һ©������ƿ���ʴ�Ϊ��������ƿ����Һ©������ƿ��
��2����ƽ��������ʱ��ѭ���������ԭ���ڸ�ʵ��ͼ�п��Կ�������ͬѧ�ڲ���ʱ��һ���������������ձ��ŷ���λ�ã�������ƽƽ��ԭ����������������=������������+���������������ŷ��ˣ���������������=������������+��������������������������=������������-��������=30g-2.6g=27.4g��
�ʴ�Ϊ���������27.4��
��3��A����ͷ�ι������Թ��л���Ⱦ�Լ���
B���Թܿ�Ӧ��������б�����������ѣ�
C��ϴ��װ�õ���Ӧ�����̳���
�ʴ�Ϊ����ͷ�ιܲ��������Թܣ��Թܿ�Ӧ��������б��ϴ��װ��Ӧ�����̳���
��4���ڷ�ˮ����μ���5-6�α���FeCl3��Һ�����������������Һ�ʺ��ɫ��ֹͣ���ȣ��ʴ�Ϊ���ڷ�ˮ����μ���5-6�α���FeCl3��aq�������������������Һ�ʺ��ɫ��ֹͣ���ȣ�
��2����ƽ��������ʱ��ѭ���������ԭ���ڸ�ʵ��ͼ�п��Կ�������ͬѧ�ڲ���ʱ��һ���������������ձ��ŷ���λ�ã�������ƽƽ��ԭ����������������=������������+���������������ŷ��ˣ���������������=������������+��������������������������=������������-��������=30g-2.6g=27.4g��
�ʴ�Ϊ���������27.4��
��3��A����ͷ�ι������Թ��л���Ⱦ�Լ���
B���Թܿ�Ӧ��������б�����������ѣ�
C��ϴ��װ�õ���Ӧ�����̳���
�ʴ�Ϊ����ͷ�ιܲ��������Թܣ��Թܿ�Ӧ��������б��ϴ��װ��Ӧ�����̳���
��4���ڷ�ˮ����μ���5-6�α���FeCl3��Һ�����������������Һ�ʺ��ɫ��ֹͣ���ȣ��ʴ�Ϊ���ڷ�ˮ����μ���5-6�α���FeCl3��aq�������������������Һ�ʺ��ɫ��ֹͣ���ȣ�
���������⿼�黯ѧʵ�鳣�������ͻ�����������Ŀ�ѶȲ���ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
 ����A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӣ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��D��Aͬ�壬����Eͬ���ڣ�EԪ�ص������������Ǵ�����������
����A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӣ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��D��Aͬ�壬����Eͬ���ڣ�EԪ�ص������������Ǵ����������� 2MgO+C
2MgO+C

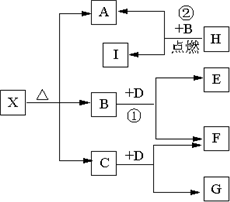
 ��(5��)��֪X��һ���Σ�H�dz����������ʣ�F��I�dz����ǽ������ʣ�E��G���ǹ�ҵ����Ҫ�ļ������ʣ���������ͼ��ʾ�Ĺ�ϵ��
��(5��)��֪X��һ���Σ�H�dz����������ʣ�F��I�dz����ǽ������ʣ�E��G���ǹ�ҵ����Ҫ�ļ������ʣ���������ͼ��ʾ�Ĺ�ϵ��