��Ŀ����
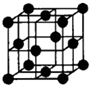
 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�8��Ԫ�أ�D��ԭ��������EС6��D��B���γ����ӻ������侧���ṹ��ͼ����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�8��Ԫ�أ�D��ԭ��������EС6��D��B���γ����ӻ������侧���ṹ��ͼ����ش���1��A��B�γɵĻ������ڹ�̬ʱ�ľ���������
���Ӿ���
���Ӿ���
��A��B�γɵĻ������A��C�γɵĻ������۵�Ҫ��
��
����ߡ��ͣ���2��д��C�ĵ�����ˮ��Ӧ�����ӷ���ʽ
Cl2+H2O=H++Cl-+HClO
Cl2+H2O=H++Cl-+HClO
����3����ͼ��ʾ��D��B�γɵ����ӻ�����Ļ�ѧʽΪ
CaF2
CaF2
����������ӻ������Ƿ�Ϊ���壬��ɿ��Ŀ�ѧ������X�������䷨
X�������䷨
�������ӻ����ᄃ����ܶ�Ϊag?cm-3��B��D��Ԫ�ص����ԭ�������ֱ�Ϊb��c�����������| 8b+4c |
| aNA |
| 8b+4c |
| aNA |
��������֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������E�����ڱ���1-18���еĵ�8��Ԫ�أ�����E��FeԪ�أ�D��ԭ��������EС6������D��CaԪ�أ�D��B���γ����ӻ�����þ����к���B����8����D���Ӹ���8��
+6��
=4�����Ըû�ѧʽΪDB2��BΪ��VIIA��Ԫ�أ�B��C��ͬһ���壬��B��ԭ������С��C������B��FԪ�أ�C��ClԪ�أ�A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壬����A��HԪ�أ�
| 1 |
| 8 |
| 1 |
| 2 |
����⣺��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������E�����ڱ���1-18���еĵ�8��Ԫ�أ�����E��FeԪ�أ�D��ԭ��������EС6������D��CaԪ�أ�D��B���γ����ӻ�����þ����к���B����8����D���Ӹ���8��
+6��
=4�����Ըû�ѧʽΪD2B��BΪ��VIIA��Ԫ�أ�B��C��ͬһ���壬��B��ԭ������С��C������B��FԪ�أ�C��ClԪ�أ�A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壬����A��HԪ�أ�
��1��HF�ǹ��ۻ�����γɵľ����Ƿ��Ӿ��壬���Ӿ���������ʵķе�������Է���������������������Ӽ��γ�����Ļ�����ʹ���ʵķе����ߣ��������к���������Ȼ����в�����������Է�����ķе�����Ȼ��⣬�ʴ�Ϊ�����Ӿ��壻�ߣ�
��2��������ˮ��Ӧ����ǿ������Ȼ����������ʴ����ᣬ���������ӷ���ʽΪ��Cl2+H2O=H++Cl-+HClO���ʴ�Ϊ��Cl2+H2O=H++Cl-+HClO��
��3��CaԪ�ظ�FԪ�ؿ��γ����ӻ�����þ����к���B������8����D���Ӹ���8��
+6��
=4�����Ըû�ѧʽΪCaF2����������ӻ������Ƿ�Ϊ���壬��ɿ��Ŀ�ѧ������X�������䷨��
ÿ�������ƾ����к���4�������ӡ�8�������ӣ�����ÿ������������Ϊ
���ܶ���ag?cm-3�����Ծ��������=
cm3=
cm3��
�ʴ�Ϊ��CaF2��X�������䷨��
��
| 1 |
| 8 |
| 1 |
| 2 |
��1��HF�ǹ��ۻ�����γɵľ����Ƿ��Ӿ��壬���Ӿ���������ʵķе�������Է���������������������Ӽ��γ�����Ļ�����ʹ���ʵķе����ߣ��������к���������Ȼ����в�����������Է�����ķе�����Ȼ��⣬�ʴ�Ϊ�����Ӿ��壻�ߣ�
��2��������ˮ��Ӧ����ǿ������Ȼ����������ʴ����ᣬ���������ӷ���ʽΪ��Cl2+H2O=H++Cl-+HClO���ʴ�Ϊ��Cl2+H2O=H++Cl-+HClO��
��3��CaԪ�ظ�FԪ�ؿ��γ����ӻ�����þ����к���B������8����D���Ӹ���8��
| 1 |
| 8 |
| 1 |
| 2 |
ÿ�������ƾ����к���4�������ӡ�8�������ӣ�����ÿ������������Ϊ
| 4M |
| NA |
| ||
| a |
| 8b+4c |
| aNA |
�ʴ�Ϊ��CaF2��X�������䷨��
| 8b+4c |
| aNA |
������������Ԫ�ص��ƶ�Ϊ���忼���˾����ļ��㡢������жϷ�����֪ʶ�㣬�ѵ��Ǽ��㾧�����ܶȣ���ȷһ�������������ǽⱾ��Ĺؼ����ѶȽϴ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�