��Ŀ����
��6�֣���֪��T��C��һ��ѹǿ�£����ݻ��ɱ���ܱ������г���2molSO2��1molO2����ʱ�����ݻ�ΪVL�����ֺ��º�ѹ��ʹ��Ӧ��2SO2��g��+O2��g��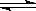 2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
 2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L��
2SO3��g����5minĩ�ﵽƽ�⣬���SO3���������Ϊ0.4���Իش��������⣺��1��ƽ����������SO2���������Ϊ ��ƽ�������O2��ת����Ϊ ����2����ѡһ�ݻ��̶�������������Կ����¶�ΪT��C������2molSO2��1molO2ʹ��Ӧ���ﵽƽ��ʱSO3�����������Ϊ0.4�����ܱ����������Ϊ�� L����1��0.4��2�֣���50%��2�֣���2��0.83V��2�֣�
��

��ϰ��ϵ�д�
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
�����Ŀ

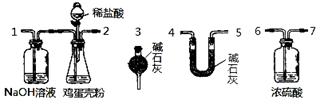
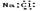

 ��100%
��100%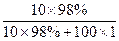 ��100%
��100%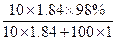 ��100%
��100% ��100%
��100%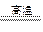 2AlN��3CO�ϳɡ�����������ȷ����
2AlN��3CO�ϳɡ�����������ȷ����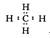
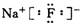
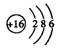
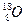

 �ṹʽΪ��H��Cl��O
�ṹʽΪ��H��Cl��O