��Ŀ����
����Ŀ�����������Ǽ�������������Ч�ɷ֡�ʵ�����Ʊ��������Ƶ�װ������ͼ����ʾ��
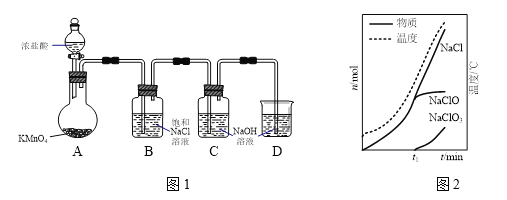
��1��װ��C�����ɸ����ʵ����ʵ�������Һ���¶���ʱ��ı仯��ͼ����ʾ��t1���Ӻ���������Ҫ��Ӧ�Ļ�ѧ����ʽΪ________________________��
��2�����Ҫ����NaClO3�����ɣ����Բ�ȡ�ķ�����_________��___________(������)��
��3����װ��C�е���Һ�õ�����������Ʒ������ô���������Ʒ�л��е�����ΪNaClO3��NaCl�е�һ�֡���ȡ2.0225 g��Ʒ����ƿ�У���ˮʹ����ȫ�ܽ⣬����Һ�м���200 mL 0.60 mol��L��1��FeSO4��Һ(����)����ַ�Ӧ��������Һ�еμ�0.50 mol��L��1��KMnO4��Һ��ǡ����ȫ��Ӧʱ����KMnO4��Һ20.00 mL���������Ʒ��NaClO����������____________��д�������������
��֪��H����ClO����Fe2����Cl����Fe3����H2O
H����ClO3-��Fe2����Cl����Fe3����H2O
H����MnO4-��Fe2����Mn2����Fe3����H2O�����Ϸ�Ӧ��δ��ƽ��
���𰸡�3Cl2��6NaOH��NaClO3��5NaCl��3H2O �����μ����� ��װ��C���ڱ�ˮ�� ����KMnO4���ʵ�����0.5 mol��L��1��0.02 L��0.01 mol ����KMnO4��Ӧ��Fe2�����ʵ�����0.01 mol��5��0.05 mol��Fe2�������ʵ�����0.60 mol��L��1��0.2 L��0.12 mol������Ʒ��Ӧ��Fe2�����ʵ�����0.12 mol��0.05 mol��0.07 mol ���������ۣ�2.0225 g��ƷȫΪNaClO��2n(NaClO)��n(Fe2��)��n(Fe2��)��2��(2.0225 g��74.5 g��mol��1)��0.0543 mol��2.0225 g��ƷȫΪNaClO3��6n(NaClO3)��n(Fe2��)��n(Fe2��)��6��(2.0225 g��106.5 g��mol��1)��0.1139 mol��2.0225 g��ƷȫΪNaCl��������Fe2��������Ʒ�к�������ΪNaClO3 ���з�����ã�74.5n(NaClO)��106.5n(NaClO3)��2.0225 g �٣�2n(NaClO)��6n(NaClO3)��0.07 mol �ڣ��ⷽ����ã�n(NaClO)��0.02 mol�� ��(NaClO)��(0.02mol��74.5 g��mol��1��2.0225g)��100%=73.67%
��������
����ͼʾ���̷������ʵ��Ʊ������롢�ᴿ�Ĺ��̣������й����ʵ����ʵ���������ؼ��㡣
(1)��ͼ��֪��Ũ�����������ط�Ӧ����Cl2�����������Ȼ�����Һ��ȥδ��Ӧ��HCl��������Cװ�������������Ʒ�Ӧ����ͼ�ҿ�֪����Ӧ���й����У��¶����ߣ�t1���Ӻ�������NaClO3����t1���Ӻ���������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��3Cl2��6NaOH��NaClO3��5NaCl��3H2O��
�ʴ�Ϊ��3Cl2��6NaOH��NaClO3��5NaCl��3H2O��
(2)��ͼ�ҿ�֪���¶�Խ�ߣ�NaClO3Խ�������ɣ�������Ҫ��СNaClO3�������ʣ����Խ��ͷ�Ӧװ�õ��¶Ȼ����HCl����μ��ٶȣ��Ӷ�����NaClO�IJ��ʣ�
�ʴ�Ϊ�������μ����ᣬ��װ��C���ڱ�ˮ�У�
(3)������֪������ƽ�ɵõ����ӷ���ʽΪ��8H����MnO4-��5Fe2��=Mn2����5Fe3����4H2O������KMnO4���ʵ�����0.5mol��L��1��0.02L��0.01 mol����KMnO4��Ӧ��Fe2�����ʵ�����0.01 mol��5��0.05mol������Ʋ�������������n(Fe2+)=0.60mol��L��1��0.2L-0.05mol��0.12 mol-0.05mol=0.07mol���ٸ��ݼ������ۣ���2.0225 g��ƷȫΪNaClO��2n(NaClO)��n(Fe2��)��n(Fe2��)��2��(2.0225g��74.5g��mol��1)��0.0543mol��2.0225g��ƷȫΪNaClO3��6n(NaClO3)��n(Fe2��)��n(Fe2��)��6��(2.0225g��106.5g��mol��1)��0.1139 mol��2.0225 g��ƷȫΪNaCl��������Fe2��������Ʒ��һ����������ΪNaClO3��ʣ��ɸ������ӷ���ʽ�ĵ�����ϵ�з����飺74.5n(NaClO)��106.5n(NaClO3)��2.0225g �٣����������Ϊ2.0225g����
2n(NaClO)��6n(NaClO3)��0.07mol��[��n(NaClO)��n(NaClO3)��ʾn(Fe2+)]���ⷽ����ã�n(NaClO)��0.02mol����(NaClO)��(0.02mol��74.5g��mol��1��2.0225g)��100%=73.67%��
�ʴ�Ϊ������KMnO4���ʵ�����0.5mol��L��1��0.02L��0.01mol ����KMnO4��Ӧ��Fe2�����ʵ�����0.01mol��5��0.05mol��Fe2�������ʵ�����0.60mol��L��1��0.2L��0.12mol������Ʒ��Ӧ��Fe2�����ʵ�����0.12mol��0.05mol��0.07mol ���������ۣ�2.0225 g��ƷȫΪNaClO��2n(NaClO)��n(Fe2��)��n(Fe2��)��2��(2.0225 g��74.5 g��mol��1)��0.0543 mol��2.0225 g��ƷȫΪNaClO3��6n(NaClO3)��n(Fe2��)��n(Fe2��)��6��(2.0225g��106.5 g��mol��1)��0.1139 mol��2.0225g��ƷȫΪNaCl��������Fe2��������Ʒ�к�������ΪNaClO3 ���з�����ã�74.5n(NaClO)��106.5n(NaClO3)��2.0225g�٣�2n(NaClO)��6n(NaClO3)��0.07 mol�ڣ��ⷽ����ã�n(NaClO)��0.02 mol����(NaClO)��(0.02mol��74.5 g��mol��1��2.0225g)��100%=73.67%��

����Ŀ����֪�������ݣ�
�� �� | �۵�/�� | �е�/�� | �ܶ�/g��cm��3 |
�� �� | ��114 | 78.4 | 0.79 |
�� �� | 16.6 | 117.9 | 1.05 |
�������� | ��83.6 | 77.5 | 0.900 |
ŨH2SO4 | 338 | 1.84 |
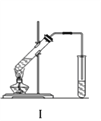

ʵ������ȡ������������Ҫװ������ͼI��ʾ����Ҫ����Ϊ������30mL�Ĵ��Թ��а������2��3��2�ı�������Ũ���ᡢ�Ҵ�������Ļ��Һ���ڰ���ͼI����װ�ã�ʹ����������������ͨ��ʢ��10mL����Na2CO3��Һ��(����2�η�̪��Һ)�Թ��У���С������Թ��еĻ��Һ���ܴ�С�Թ����ռ�Լ2mL����ʱֹͣ���ȣ�����С�Թܲ�������Ȼ���ã��ݷ����������������������ش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ�����_____________________________��
��2��д����ʵ����ȡ���������Ļ�ѧ����ʽ_________________________________��ŨH2SO4�������� _______________________��
��3��������У���С������Թ��еĻ��Һ����ԭ��_________________________��
��4����������۲쵽��������___________________________________________________
��5��������У���������������ķ�����_________________________________��
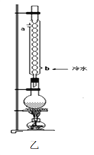
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ����������ͼ�ס��ҵ�װ��(��ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡԲ����ƿ�в���)������Ϊ����װ�ø�������Ϊʲô��_____��