��Ŀ����
��������ʱ������(N2H4)Ϊȼ�ϣ���NO2�������������߷�Ӧ���ɵ�����ˮ��������֪��N2(g)��2O2(g)��2NO2(g)����H����67.7 kJ��mol��1
N2H4(g)��O2(g)![]() N2(g)��2H2O(g)����H����534 kJ��mol��1
N2(g)��2H2O(g)����H����534 kJ��mol��1
(1)д���ºͶ���������Ӧ���Ȼ�ѧ����ʽ________��
(2)��֪1 molҺ̬ˮ����ʱ������44 kJ����������16 g�ºͶ���������ȫ��Ӧ����Һ̬ˮʱ�ų�������Ϊ________kJ��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
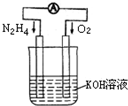 ���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮
���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮