��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣��û�ѧ����ش��������⣺
��1��������A���ɢݢ�����Ԫ�ع��ɵģ��õ���ʽ��ʾ���γɹ���______________
��2���ܡ���Ԫ���γɵĻ������У��ߵ�ԭ������ܡ���Ļ�ѧ������֮��___________________
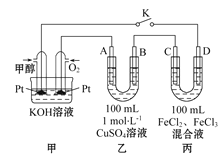
��3���ڵ�����⻯��������������Ƴ�ȼ�ϵ�أ�д���ڼ��������¸����ĵ缫����ʽ_____________________________________
��4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
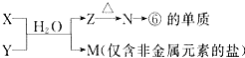
a���ĵ�����ݵ�����������Ӧˮ�����ˮ��Һ��Ӧ�����ӷ���ʽΪ��___________________��
b��M��������ѧ��������___________________________________��
c��M�е������ӵļ���������_______________________________________��
���𰸡�![]() 1:4 CH4-8e-+10OH-=CO32-+7H2O 2 Al + 2OH- + 2H2O=2AlO2- + 3H2�� ���Ӽ����ۼ� ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+
1:4 CH4-8e-+10OH-=CO32-+7H2O 2 Al + 2OH- + 2H2O=2AlO2- + 3H2�� ���Ӽ����ۼ� ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+
��������
��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����Ϊ��
��1��������A���ɢݢ�����Ԫ�ع��ɵģ���Ϊ�Ȼ��ƣ��û�����Ϊ���ӻ����Na��Cl֮��ͨ����ʧ���Ӷ��γ����Ӽ����ݴ�����
��2������������һ����ԭ�ӿ��γ�4������������
��3���������ȼ�ϵ���У���������ʧ���ӣ�����������Ӧ��
��4��a���ĵ���ΪAl���ݵ�����������Ӧˮ����ΪNaOH�����߷�Ӧ����ƫ��������������
b��MΪ�Ȼ�泥�Ϊ���ӻ����
c��M�е�������Ϊ笠����ӣ����ݳ��������ӵļ��鷽����������
��1������A���ɢݢ�����Ԫ�ع��ɵģ���ΪNaCl�������ʽ��ʾ���γɹ���Ϊ��
![]() ��
��
�ʴ�Ϊ��![]() ��
��
��2����ΪO����ΪSi���������γɵĻ�����Ϊ�������裬�û�����Ϊԭ�Ӿ��壬�侧���ڲ�һ����ԭ�ӿ��γ�4��Si-O�������ԭ�Ӹ����뻯ѧ��֮��Ϊ1:4��
�ʴ�Ϊ��1��4��
��3���ڵ�����⻯��Ϊ���飬������������Ƴɼ���ȼ�ϵ�أ��为����ӦʽΪ��CH4-8e-+10OH-=CO32-+7H2O��
�ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��
��4��a���ĵ���ΪAl���ݵ�����������Ӧˮ����ΪNaOH���䷴Ӧ�����ӷ���ʽΪ��2Al + 2OH- + 2H2O=2AlO2- + 3H2����
�ʴ�Ϊ��2Al + 2OH- + 2H2O=2AlO2- + 3H2����
b��MΪ�����ǽ���Ԫ�ص��Σ������֪MΪ�Ȼ�泥��仯ѧ������Ϊ���Ӽ����ۼ���
�ʴ�Ϊ�����Ӽ����ۼ���
c��M�е�������Ϊ笠����ӣ�����鷽��Ϊ��ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+��
�ʴ�Ϊ��ȡ��Ʒ���Թ��У���������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ�����Ĵ̼�����ζ���壬˵������NH4+��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ���������л�ѧʵ��ʾ��ͼ������ʵ�����������Ϸ����ó��Ľ�����ȷ����
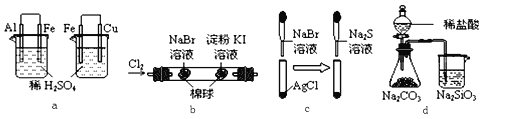
ѡ�� | ʵ�� | ʵ������ | ���� |
A | a | ���ձ��������������ݣ����ձ���ͭ���������� | ��ԣ�Al��Fe��Cu |
B | b | �������Ϊ��ɫ���ұ�����Ϊ��ɫ | �����ԣ�Cl2��Br2��I2 |
C | c | ��ɫ�����ȱ�Ϊ����ɫ�����Ϊ��ɫ | �ܽ��:AgCl < AgBr < Ag2S |
D | d | ��ƿ��������������ձ���Һ������ | �ǽ����ԣ�Cl��C��Si |
A. A B. B C. C D. D