��Ŀ����
 ij�о���ѧϰС��Ϊ֤��2Fe3++2I-?2Fe2++I2Ϊ���淴Ӧ������Ӧ����һ�����ȣ���������¼��ַ�������֪FeF63-��һ����ɫ���ȶ��������ӣ�
ij�о���ѧϰС��Ϊ֤��2Fe3++2I-?2Fe2++I2Ϊ���淴Ӧ������Ӧ����һ�����ȣ���������¼��ַ�������֪FeF63-��һ����ɫ���ȶ��������ӣ���Ҫ��ش��������⣮
�����ף�
ȡ5mL 0.1mol/L KI��Һ���μ�2ml 0.1mol/L ��FeCl3��Һ���ټ�������2mL CCl4����������á��ֲ㣬��ȡ�ϲ���Һ���μ�KSCN��Һ��
��1����������֤���÷�ӦΪ���淴Ӧ��������
��2����ͬѧ��Ϊ�÷�����Ʋ������ܣ���ʹ�÷�ӦΪ�����淴ӦҲ���ܳ�������������ԭ����
�����ң�
ȡ5mL 0.1mol/L KI��Һ���μ�2ml 0.1mol/L ��FeCl3��Һ����Һ���ػ�ɫ��������Һ�еμ�NH4F��Һ��������
��������
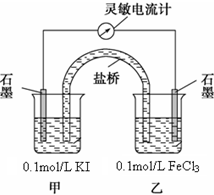
�����ͼԭ���װ�ã���ͨ���������ƣ�ָ������ƫת��ע������������ָ������ƫ���Դ������������ʱ����е����ƶ�����С����������Ϊ�㣮
��ָ���������������ձ��м���1mol/L FeCl2��Һ�����۲쵽���������Ƶ�ָ����
�����������ף���1����÷�ӦΪ���淴Ӧ���������Ȼ�̼�����Ȼ�̼����Ϻ�ɫ���ϲ���Һ�еμ�KSCN����Һ��Ѫ��ɫ��
��2���������У�Fe2+�����ױ���������������Fe3+��
�����ң�����Һ�еμ�NH4F��Һ��������Ӧ������ɫ��FeF63-������Һ��Ϊ��ɫ��˵��ƽ�ⷢ���ƶ���
��������ͼ�����������Ƶ�ָ��ָ���ң��Ҳ��ձ�Ϊ��������ָ���������������ձ��м���1mol/L FeCl2��Һ����Ϊ���淴Ӧ���ɷ���2Fe2++I2?2Fe3++2I-��I2����ԭ��ָ��Ӧƫ����
��2���������У�Fe2+�����ױ���������������Fe3+��
�����ң�����Һ�еμ�NH4F��Һ��������Ӧ������ɫ��FeF63-������Һ��Ϊ��ɫ��˵��ƽ�ⷢ���ƶ���
��������ͼ�����������Ƶ�ָ��ָ���ң��Ҳ��ձ�Ϊ��������ָ���������������ձ��м���1mol/L FeCl2��Һ����Ϊ���淴Ӧ���ɷ���2Fe2++I2?2Fe3++2I-��I2����ԭ��ָ��Ӧƫ����
����⣺�����ף���1����÷�ӦΪ���淴Ӧ���������Ȼ�̼�����Ȼ�̼����Ϻ�ɫ���ϲ���Һ�еμ�KSCN����Һ��Ѫ��ɫ��
�ʴ�Ϊ���²㣨CCl4�㣩��Һ���Ϻ�ɫ�����ϲ���Һ�еμ�KSCN����Һ��Ѫ��ɫ��
��2���������У�Fe2+�����ױ���������������Fe3+������֤����Ӧ���棬
�ʴ�Ϊ�������в����е�Fe2+���ӱ�����������������
�����ң�����Һ�еμ�NH4F��Һ��������Ӧ������ɫ��FeF63-������Һ��Ϊ��ɫ��˵�����淴Ӧ����
�ʴ�Ϊ����Һ���ػ�ɫ��Ϊ��ɫ�����dz����Fe3+��F-���������ɫ��FeF63-����ʹ2Fe3++2I-?2Fe2++I2ƽ�⳯�淴Ӧ�����ƶ���
��������ͼ�����������Ƶ�ָ��ָ���ң��Ҳ��ձ�Ϊ��������ָ���������������ձ��м���1mol/L FeCl2��Һ����Ϊ���淴Ӧ���ɷ���2Fe2++I2?2Fe3++2I-��I2����ԭ��ָ��Ӧƫ������ʯī�缫�ϵĵ缫��ӦʽΪI2+2e-=2I-��
�ʴ�Ϊ����I2+2e-=2I-��
�ʴ�Ϊ���²㣨CCl4�㣩��Һ���Ϻ�ɫ�����ϲ���Һ�еμ�KSCN����Һ��Ѫ��ɫ��
��2���������У�Fe2+�����ױ���������������Fe3+������֤����Ӧ���棬
�ʴ�Ϊ�������в����е�Fe2+���ӱ�����������������
�����ң�����Һ�еμ�NH4F��Һ��������Ӧ������ɫ��FeF63-������Һ��Ϊ��ɫ��˵�����淴Ӧ����
�ʴ�Ϊ����Һ���ػ�ɫ��Ϊ��ɫ�����dz����Fe3+��F-���������ɫ��FeF63-����ʹ2Fe3++2I-?2Fe2++I2ƽ�⳯�淴Ӧ�����ƶ���
��������ͼ�����������Ƶ�ָ��ָ���ң��Ҳ��ձ�Ϊ��������ָ���������������ձ��м���1mol/L FeCl2��Һ����Ϊ���淴Ӧ���ɷ���2Fe2++I2?2Fe3++2I-��I2����ԭ��ָ��Ӧƫ������ʯī�缫�ϵĵ缫��ӦʽΪI2+2e-=2I-��
�ʴ�Ϊ����I2+2e-=2I-��
���������⿼�黯ѧ��Ӧԭ����̽������Ŀ������ѧ�����������ʵ�������Ŀ��飬�Ѷ��еȣ�ע��ʵ��ԭ���������Ե��жϣ�

��ϰ��ϵ�д�
 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
�����Ŀ
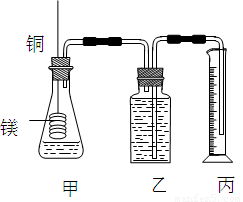
 ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨ��ʵ�������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨ��ʵ�������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£� ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ��Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ��Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�