��Ŀ����
����Ŀ�����⻯�ƣ� NaAlH4�����л��ϳɵ���Ҫ��ԭ��������������Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3�����ʣ�Ϊԭ���Ʊ����⻯�Ƶ�һ�ֹ�����������ͼ��ʾ��

ע��SiO2�ڡ����ܡ�ʱת��Ϊ�������ƣ�Na2Al2SixO8��������
��1���������ƣ�Na2 Al2SixO4�����������������ʽ��ʾ����ɣ���ʽΪ_______��
��2��������I������������Ҫ�ɷ���_______�������ƣ������ˢ�������Һ�м���NaHCO3��Һ��������Ӧ�����ӷ���ʽΪOH����HCO3��===CO32����H2O��__________________________��
��3�������I������һ������1000��ʱ����N2��Ӧ�Ʊ�AIN�������ֲ�������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ�������Ҫԭ����___________��
��4���������ǵ��Na2CO3��Һ����ԭ����ͼ��ʾ��
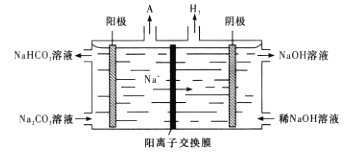
�����ĵ缫��ӦʽΪ____________����֪F��96500C��mol����I��0.5Aʱ��ͨ��80min���������������H2����״���£������Ϊ_____L�����������λ��Ч���֣���
��5�����⻯����ˮ�������ҷ�Ӧ���ҷ�Ӧ�����������ݣ��䷴Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
���𰸡�Na2O��Al2O3��2SiO2 �������ơ������� A1O2����HCO3����H2O=CO32��+Al(OH)3�� �Ȼ�立ֽ�������Ȼ����ܹ��ƻ����������������Ĥ 4CO32����2H2O��4e��=4HCO3����O2�� 0.28 NaAlH4+2H2O NaAlO2��4H2��
��������
����������Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3�����ʣ�Ϊԭ���Ʊ����⻯�ƣ������̿�֪����NaOH�ܽ�ʱFe2O3����Ӧ������Ϣ��֪SiO2����������ʱת��Ϊ�������Ƴ��������˵õ�������ΪFe2O3���������ƣ�̼��������ƫ�����ɷ�Ӧ����Al(OH)3������II�õ�Al(OH)3���������������������IΪ�������������Al�����������IIΪ���Na2CO3��Һ�����ͼ��֪��������̼�������ʧȥ��������̼��������Ӻ������������������ӵõ���������������
��1���������ƣ�Na2Al2SixO8����Na��Al��Si��O�Ļ��ϼ۷ֱ�Ϊ+1��+3��+4��-2�����ݻ��������������ϼ۴�����Ϊ0����1��2+3��2+4x-2��8=0�����x=2������������ΪNa2Al2Si2O8�����������������ʽ��ʾΪNa2O��Al2O3��2SiO2���ʴ�Ϊ��Na2O��Al2O3��2SiO2��
��2����������������������I����������Ҫ���������ơ�����������������I��������Һ�м���NaHCO3��Һ��̼��������ƫ�����ƺ������������Ʒ�Ӧ����Ӧ�����ӷ���ʽΪOH����HCO3��=CO32����H2O��A1O2����HCO3����H2O=CO32��+Al(OH)3�����ʴ�Ϊ���������ơ���������AlO2����HCO3����H2O=CO32��+Al(OH)3����
��3��������1000��ʱ����N2��Ӧ�Ʊ�AlN������������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ�������Ҫԭ����NH4Cl�ֽ������HCI�ܹ��ƻ�Al�����Al2O3��Ĥ��ʹ���������뵪����Ӧ���ʴ�Ϊ��NH4Cl�ֽ������HC�ܹ��ƻ�Al�����Al2O3��Ĥ��
��4����ͼ��֪��������ӦΪ4CO32����2H2O��4e��=4HCO3����O2���������������ӵõ�����������������֪F��96500C��mol����I��0.5Aʱ��ͨ��80min������Ϊ0.5A��4800s=2400C��ת�Ƶ��ӵ����ʵ���Ϊ��![]() 0.02487mol���������������H2����״���£������Ϊ��0.02487mol ��0.5��22.4L/mol
0.02487mol���������������H2����״���£������Ϊ��0.02487mol ��0.5��22.4L/mol![]() ���ʴ�Ϊ��4CO32����2H2O��4e��=4HCO3����O2����0.28��
���ʴ�Ϊ��4CO32����2H2O��4e��=4HCO3����O2����0.28��
��5�����⻯�ƣ�NaAlH4����ˮ�������ҷ�Ӧ�����������ݣ���Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��NaAlH4+2H2O=NaAlO2��4H2�����ʴ�Ϊ��NaAlH4+2H2O NaAlO2��4H2��

����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⣺
(1)��֪Ԫ��M���������Ca5(PO4)3F��һ��Ԫ�ء�Ԫ��M����̬ԭ�����ʧȥ��1������5��������������(�������ܣ��÷���I1��I5��ʾ)�����ʾ��
I1 | I2 | I3 | I4 | I5 | |
������ | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 |
Ԫ��M����̬�������ϼ���_________�ۣ����̬ԭ�ӵ����Ų�ʽΪ_________��
(2)Ca3(PO4)3F�зǽ���Ԫ�ص縺���ɴ�С��˳��Ϊ_________��
(3)PO43-������ԭ�ӵ��ӻ���ʽΪ_________�������ӵĿռ乹��Ϊ_________������Ϊ________����ȵ�������_________ (��д������)��
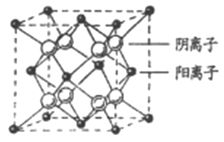
(4)CaF2�����ṹ��ͼ��ʾ����CaF2��������Ca2+����ҵȾ����Ca2+��ĿΪ_________����֪Ca2+��F�뾶�ֱ�Ϊa cm��b cm�������ӵ�����ΪNA��MΪĦ�������������ܶ�Ϊ________g��cm3(���ػ���)��
(5)��֪MgO��CaO�ľ���ṹ���ƣ���Ħ��Ӳ�ȵĴ�С��ϵΪ_________��ԭ��Ϊ___________��
����Ŀ����ͼ��ʾװ��(����������)�����Ƚ�����Һ�������У��۲쵽�������Թ���һ��ʱ���ָ�ԭ״���ٽ�����Һ����붡�У����������Թ������������ȷ���ǣ� ��

ѡ�� | �ιܼ� | �ձ��� | �ι��� | �ձ��� |
A | ˫��ˮ | �������� | ˮ | �������� |
B | ϡ���� | þ | ���� | ̼��� |
C | ˮ | �������� | ˮ | ����� |
D | ˮ | ������ | ���� | þ |
A.AB.BC.CD.D


