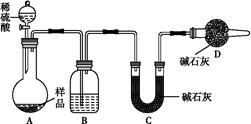
ΧβΡΩΡΎ»ί
Έ“ΙζΜ·ΙΛΉ®Φ“ΚνΒ¬ΑώΘ§”¬”Ύ¥¥–¬Θ§ΗΡΫχΑ±ΦνΖ®…ηΦΤΝΥΓΑΝΣΚœ÷ΤΦνΖ®Γ±Θ§ΈΣ άΫγ÷ΤΦνΙΛ“ΒΉς≥ωΝΥΆΜ≥ωΙ±œΉΓΘ«κΆξ≥…œ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΓΑΝΣΚœ÷ΤΦνΖ®Γ±÷ΤΒΟΒΡΓΑΦνΓ± « (ΧνΜ·―ß Ϋ)ΓΘ
Θ®2Θ©Α±ΦνΖ®ΚΆΝΣΚœ÷ΤΦνΖ® «ΝΫ¥σ÷Ί“ΣΒΡΙΛ“Β÷ΤΦνΖ®Θ§œ¬Ν–±μ¥ο÷–Θ§≤Μ’ΐ»ΖΒΡ « ΓΘ
| | | Α±ΦνΖ® | ΝΣΚœ÷ΤΦνΖ® |
| A | ‘≠Νœ | ≥―ΈΓΔΑ±ΤχΓΔ…ζ ·Μ“ | ≥―ΈΓΔΑ±ΤχΓΔΕΰ―θΜ·ΧΦ |
| B | Ω…ΡήΒΡΗ±≤ζΈο | ¬»Μ·ΗΤ | ¬»Μ·οß |
| C | ―≠ΜΖΈο÷ | Α±ΤχΓΔΕΰ―θΜ·ΧΦ | ¬»Μ·ΡΤ |
| D | ΤάΦέ | ‘≠Νœ“ΉΒΟΘΜ…η±ΗΗ¥‘”ΘΜΡήΚΡΗΏ | ‘≠Νœάϊ”Ο¬ ΗΏΘΜΖœΤζΈο…Ό |
Ρ≥ Β―ι–ΓΉιΘ§άϊ”Οœ¬Ν–ΉΑ÷ΟΡΘΡβΓΑΝΣΚœ÷ΤΦνΖ®Γ±ΓΘ

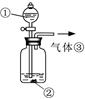
Θ®3Θ©»Γ…œ ω“«ΤςΝ§Ϋ”ΉΑ÷ΟΘ§Υ≥–ρΈΣΘΚ(a)Ϋ”( )ΓΔ( )Ϋ”( )ΘΜ(b)Ϋ”( )ΘΜ
Φλ―ιΤχΟή–‘ΚσΉΑ»κ“©ΤΖΘ§”ΠΗΟœ»»Ο ΉΑ÷ΟΘ®Χν…œ ωΉ÷ΡΗΘ©œ»ΖΔ…ζΖ¥”ΠΘ§÷±ΒΫ≤ζ…ζΒΡΤχΧε≤ΜΡή‘Ό‘ΎC÷–»ήΫβ ±Θ§‘ΌΆ®»κΝμ“ΜΉΑ÷Ο÷–≤ζ…ζΒΡΤχΧεΓΘ
Θ®4Θ©C÷–”Ο«ρ–ΈΗ…‘οΙήΕχ≤Μ”Ο÷±ΒΦΙήΘ§ΤδΉς”Ο « Θ§D÷–”Π―Γ”ΟΒΡ“ΚΧεΈΣ ΘΜ
Θ®5Θ©C÷–ΙψΩΎΤΩΡΎ≤ζ…ζΙΧΧεΒΡΉήΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
Θ®6Θ©≤ζΤΖ¥ΩΦν÷–Κ§”–ΧΦΥα«βΡΤΓΘ»γΙϊ”ΟΦ”»»Ζ÷ΫβΒΡΖΫΖ®≤βΕ®¥ΩΦν÷–ΧΦΥα«βΡΤΒΡ÷ ΝΩΖ÷ ΐΘ§¥ΩΦν÷–ΧΦΥα«βΡΤΒΡ÷ ΝΩΖ÷ ΐΩ…±μ ΨΈΣΘΚ (ΉΔΟςΡψΒΡ±μ¥ο Ϋ÷–Υυ”ΟΒΡ”–ΙΊΖϊΚ≈ΒΡΚ§“ε)ΓΘ
Θ®1Θ©Na2CO3
Θ®2Θ©A.C
Θ®3Θ©f e d c Θ®2Ζ÷Θ© B
Θ®4Θ©ΖάΒΙΈϋ ±ΞΚΆNaHCO3»ή“Κ
Θ®5Θ©CO2+NH3+NaCl+H2OΓζNaHCO3Γΐ+NH4Cl
Θ®6Θ©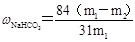 Θ® m1ΈΣ―υΤΖ÷ ΝΩΘ§m2ΈΣΦ”»»Κσ≤ζΤΖΒΡ÷ ΝΩΘ©
Θ® m1ΈΣ―υΤΖ÷ ΝΩΘ§m2ΈΣΦ”»»Κσ≤ζΤΖΒΡ÷ ΝΩΘ©
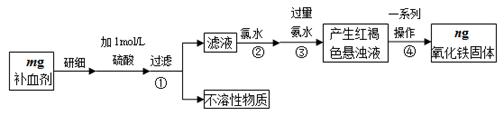
ΫβΈω ‘ΧβΖ÷ΈωΘΚΘ®1Θ©ΓΑΝΣΚœ÷ΤΦνΖ®Γ±÷ΤΒΟΒΡΓΑΦνΓ± «ΧΦΥαΡΤΘ§Μ·―ß ΫΈΣNa2CO3ΓΘ
Θ®2Θ©AΓΔΑ±ΦνΖ®‘≠Νœ”–ΘΚ ≥―ΈΘ®¬»Μ·ΡΤΘ©ΓΔ ·Μ“ ·Θ®Ψ≠λ―…’…ζ≥……ζ ·Μ“ΚΆΕΰ―θΜ·ΧΦΘ©ΓΔΑ±ΤχΘ§ΝΣΚœ÷ΤΦνΖ®‘≠Νœ”–ΘΚ ≥―ΈΓΔΑ±ΤχΓΔΕΰ―θΜ·ΧΦΘ§Ι A¥μΈσΘΜBΓΔΑ±ΦνΖ®Ω…ΡήΒΡΗ±≤ζΈοΈΣ¬»Μ·ΗΤΘ§ΝΣΚœ÷ΤΦνΖ®Ω…ΡήΒΡΗ±≤ζΈο¬»Μ·οßΘ§Ι B’ΐ»ΖΘΜCΓΔΑ±ΦνΖ®―≠ΜΖΈο÷ ΘΚΑ±ΤχΓΔΕΰ―θΜ·ΧΦΘ§ΝΣΚœ÷ΤΦνΖ®―≠ΜΖΈο÷ ΘΚ¬»Μ·ΡΤΘ§Εΰ―θΜ·ΧΦΘ§Ι C¥μΈσΘΜDΓΔΑ±ΦνΖ®‘≠ΝœΘ® ≥―ΈΚΆ ·Μ“ ·Θ©±ψ“ΥΘ§≤ζΤΖ¥ΩΦνΒΡ¥ΩΕ»ΗΏΘ§Η±≤ζΤΖΑ±ΚΆΕΰ―θΜ·ΧΦΕΦΩ…“‘ΜΊ ’―≠ΜΖ Ι”ΟΘ§÷Τ‘λ≤Ϋ÷ηΦρΒΞΘ§ Κœ”Ύ¥σΙφΡΘ…ζ≤ζΘ§ΒΪ…η±ΗΗ¥‘”ΘΜΡήΚΡΗΏΘ§Α±ΦνΖ®ΒΡΉν¥σ»±ΒψΜΙ‘Ύ”Ύ‘≠Νœ ≥―ΈΒΡάϊ”Ο¬ ÷Μ”–72%ΓΪ74%ΘΜΝΣΚœ÷ΤΦνΖ®Ήν¥σΒΡ”≈Βψ « Ι ≥―ΈΒΡάϊ”Ο¬ ΧαΗΏΒΫ96%“‘…œΘ§ΖœΤζΈο…ΌΘ§Ι D’ΐ»ΖΘ§¥πΑΗ―ΓACΓΘ
Θ®3Θ©AΉΑ÷Ο «÷Τ±ΗCO2Θ§BΉΑ÷Ο «÷Τ±ΗNH3Θ§”…”ΎΑ±ΤχΦΪ“Ή»ή”ΎΥ°–η“Σ”ΟΗ…‘οΙήΖά÷ΙΒΙΈϋΘ§ΝμΆβCO2÷–ΒΡ¬»Μ·«βΤχΧεΜΙ–η“Σ≥ΐ»ΞΘ§“ρ¥Υ’ΐ»ΖΒΡΝ§Ϋ”Υ≥–ρΈΣ(a)Ϋ”(f)ΓΔ(e)Ϋ”(d)ΘΜ(b)Ϋ”(c)ΓΘ”…”ΎCO2‘ΎΥ°÷–ΒΡ»ήΫβΕ»–ΓΘ§“ρ¥Υ“Σ Ήœ»≤ζ…ζΑ±ΤχΘ§»ΜΚσ‘ΌΆ®»κCO2ΤχΧεΘ§Υυ“‘”ΠΗΟœ»»ΟBΉΑ÷Οœ»ΖΔ…ζΖ¥”ΠΓΘ
Θ®4Θ©Α±ΤχΦΪ“Ή»ή”ΎΥ°Θ§“ρ¥ΥC÷–”Ο«ρ–ΈΗ…‘οΙήΕχ≤Μ”Ο÷±ΒΦΙήΒΡΉς”Ο «ΖάΒΙΈϋΓΘ≥ΐ»ΞCO2÷–ΒΡ¬»Μ·«βΤχΧε”ΠΗϔϱΞΚΆNaHCO3»ή“ΚΘ§Φ¥D÷–”Π―Γ”ΟΒΡ“ΚΧεΈΣ±ΞΚΆNaHCO3»ή“ΚΓΘ
Θ®5Θ©CΉΑ÷Ο «÷Τ±ΗΧΦΥα«βΡΤΒΡΘ§Υυ“‘C÷–ΙψΩΎΤΩΡΎ≤ζ…ζΙΧΧεΒΡΉήΜ·―ßΖΫ≥Χ ΫΈΣCO2+NH3+NaCl+H2OΓζNaHCO3Γΐ+NH4ClΓΘ
Θ®6Θ©…ηm1ΈΣ―υΤΖ÷ ΝΩΘ§m2ΈΣΦ”»»Κσ≤ζΤΖΒΡ÷ ΝΩΘ§‘ρΗυΨίΧΦΥα«βΡΤΖ÷ΫβΒΡΖΫ≥Χ ΫΩ…÷Σ
2NaHCO3 Na2CO3ΘΪH2OΘΪCO2Γϋ ΙΧΧε÷ ΝΩΦθ…ΌΓςm
Na2CO3ΘΪH2OΘΪCO2Γϋ ΙΧΧε÷ ΝΩΦθ…ΌΓςm
168g 106g 62g
X m1Θ≠m2
ΫβΒΟXΘΫ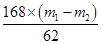
Υυ“‘¥ΩΦν÷–ΧΦΥα«βΡΤΒΡ÷ ΝΩΖ÷ ΐΩ…±μ ΨΈΣ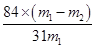 ΓΘ
ΓΘ
ΩΦΒψΘΚΩΦ≤ιΚν œ÷ΤΦνΖ®ΒΡ”–ΙΊ≈–ΕœΓΔΦΤΥψΒ»

 ΉέΚœΉ‘≤βœΒΝ–¥πΑΗ
ΉέΚœΉ‘≤βœΒΝ–¥πΑΗΘ®1Θ¥Ζ÷Θ©
“ΜΈΜΆ§―ß‘ΎΗ¥œΑ ±”ωΒΫ’β―υ“ΜΒάœΑΧβΘΚΡ≥Έό…Ϊ»ή“Κ÷–Ω…ΡήΚ§”–ΓΑH+ΓΔOH-ΓΔNa+ΓΔNO3-Γ±Θ§Φ”»κ¬ΝΖέΚσΘ§÷Μ≤ζ…ζH2Θ§Έ ΗΟΈό…Ϊ»ή“Κ÷–Ρή¥σΝΩ¥φ‘ΎΡΡΦΗ÷÷άκΉ”ΓΘ
Θ®1Θ©Φ”»κ¬ΝΖέ≤ζ…ζH2Θ§ΥΒΟς¬ΝΨΏ”–______Θ®ΧνΓΑ―θΜ·–‘Γ±ΜρΓΑΜΙ‘≠–‘Γ±Θ©ΓΘ
Θ®2Θ©ΗΟΆ§―ßΖ÷ΈωΘΚ»τH+¥σΝΩ¥φ‘ΎΘ§‘ρNO3-ΨΆ≤ΜΡή¥σΝΩ¥φ‘ΎΓΘ
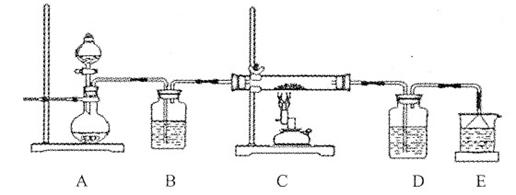
…ηΦΤ Β―ι÷Λ Β»γœ¬ΘΚ
| ΉΑ ÷Ο | œ÷ œσ |
 | ΔΓ. Β―ι≥θ ΦΘ§Έ¥ΦϊΟςœ‘œ÷œσ ΔΔ. Ιΐ“ΜΜαΕυΘ§≥ωœ÷Τχ≈ίΘ§“ΚΟφ…œΖΫ≥ «≥ΉΊ…Ϊ ΔΘ. ‘Ιή±δ»»Θ§»ή“ΚΖ–ΧΎ |
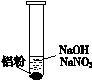
ΔΌ ―ΈΥα»ήΫβAl2O3±ΓΡΛΒΡάκΉ”ΖΫ≥Χ Ϋ «______ΓΘ
ΔΎ ΗυΨίœ÷œσΔΔΘ§ΆΤ≤β»ή“Κ÷–≤ζ…ζΝΥNOΘ§ΈΣΫχ“Μ≤Ϋ»Ζ»œΘ§Ϋχ––»γœ¬ Β―ιΘΚ
| Β ―ι | ΡΎ »ί | œ÷ œσ |
| Β―ι1 | ΫΪ Σ»σKIΓΣΒμΖέ ‘÷Ϋ÷Ο”ΎΩ’Τχ÷– | Έ¥±δάΕ |
| Β―ι2 | ”Ο Σ»σKIΓΣΒμΖέ ‘÷ΫΦλ―ι«≥ΉΊ…ΪΤχΧε | ‘÷Ϋ±δάΕ |
b. Β―ι1ΒΡΡΩΒΡ «_______ΓΘ
c. Β―ι1ΓΔ2ΥΒΟςΖ¥”Π…ζ≥…ΝΥNOΘ§ΫΪ…ζ≥…NOΒΡάκΉ”ΖΫ≥Χ Ϋ≤Ι≥δΆξ’ϊΘΚ
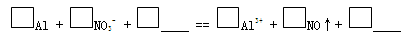
Θ®3Θ©‘ΌΦΌ…ηΘΚ»τOH-¥σΝΩ¥φ‘ΎΘ§NO3-“≤Ω…Ρή≤ΜΡή¥σΝΩ¥φ‘ΎΓΘ
÷Ί–¬…ηΦΤ Β―ι÷Λ Β»γœ¬ΘΚ
| ΉΑ ÷Ο | œ÷ œσ |
 | ΔΓ. Β―ι≥θ ΦΘ§Έ¥ΦϊΟςœ‘œ÷œσ ΔΔ. Ιΐ“ΜΜαΕυΘ§≥ωœ÷Τχ≈ίΘ§”–¥ΧΦΛ–‘ΤχΈΕ |
ΈΣ»Ζ»œΓΑ¥ΧΦΛ–‘ΤχΈΕΓ±ΤχΧεΘ§Ϋχ––»γœ¬ Β―ιΘΚ”Ο Σ»σKIΓΣΒμΖέ ‘÷ΫΦλ―ιΘ§Έ¥±δάΕΘΜ”Ο Σ»σΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§ ‘÷Ϋ±δάΕΓΘ
ΔΌ ¥ΧΦΛ–‘ΤχΈΕΒΡΤχΧε «______ΓΘ
ΔΎ ≤ζ…ζΗΟΤχΧεΒΡάκΉ”ΖΫ≥Χ Ϋ «______ΓΘ
Θ®4Θ©‘ΎNaOH»ή“Κ÷–Φ”»κ¬ΝΖέΘ§ΫαΙϊ÷ΜΦλ―ι≥ω”–H2…ζ≥…Θ§ΤδΜ·―ßΖΫ≥Χ Ϋ «______ΓΘ
Θ®5Θ© Β―ιΫαΙϊ÷Λ ΒΘΚNO3?‘ΎΥαΓΔΦν–‘ΜΖΨ≥÷–ΕΦ”–“ΜΕ®ΒΡ―θΜ·–‘Θ§Ρή―θΜ·¬ΝΒΞ÷ Θ§≤ζ…ζΚ§ΒΣΜ·ΚœΈοΓΘœΑΧβ÷–ΒΡΈό…Ϊ»ή“Κ“ΜΕ®Ρή¥σΝΩ¥φ‘ΎΒΡ «Na+ΚΆOH-ΓΘ
Β―ιΡΩΒΡΘΚΧΫΨΩΙΐ―θΜ·ΡΤ”κΥ°Ζ¥”ΠΚσΒΡ»ή“ΚΒΈΦ”Ζ”ΧΣ ‘“Κœ»±δΚλΚσΆΥ…ΪΒΡ‘≠“ρΓΘ
[Ζ÷Έω”κ≤¬œκ]
Θ®1Θ©ΗυΨίΙΐ―θΜ·ΡΤ”κΥ°Ζ¥”ΠΒΡ‘≠άμΘΚ2Na2O2 + 2H2O =" 4NaOH" + O2ΓϋΘ§ΆυΙΐ―θΜ·ΡΤΙΧΧεΆξ»Ϊ»ήΫβΖ¥”ΠΚσΒΡ»ή“Κ÷–ΒΈΦ”Ζ”ΧΣ±Ψ”Π÷ΜΜα±δΚλΕχ≤ΜΜαΆΥ…ΪΘ§Εχ Β―ι÷–ΖΔœ÷Ζ”ΧΣ±δΚλΚσ”÷ΆΥ…ΪΓΘ”…¥ΥΧα≥ω»γœ¬ΒΡ≤¬œκΘΚ
AΘ°―θΤχ”–Τ·ΑΉ–‘
BΘ°«β―θΜ·ΡΤ”–Τ·ΑΉ–‘
CΘ°
[ Β―ι”κ≈–Εœ] «κΆξ≥…œ¬Ν–±μΗώΘΚ
| Β―ι±ύΚ≈ | 1 | 2 | 3 |
| Β―ιΉΑ÷Ο | 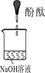 | 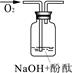 | 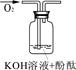 |
| ―ι÷Λ≤¬œκ | | C | |
| Β―ιœ÷œσ | »ή“Κ±δΚλΚσ≤ΜΆΥ…Ϊ | ||
| Β―ιΥΒΟς | 1ΓΔ2ΒΡ Β―ι÷–NaOH»ή“Κ «”Ο Θ®ΧνΓΑ«β―θΜ·ΡΤΙΧΧεΓ±ΓΔΓΑ―θΜ·ΡΤΙΧΧεΓ±ΓΔΓΑΙΐ―θΜ·ΡΤΙΧΧεΓ±Θ©»ή”ΎΥ°≈δ÷ΤΒΡΓΘ | ||
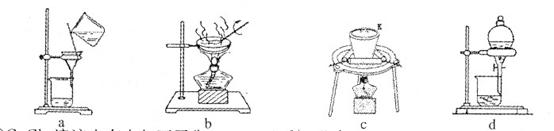
Θ®2Θ©ΗυΨί“‘…œ Β―ιΖ÷ΈωΖΔœ÷ΘΚΙΐ―θΜ·ΡΤ”κΥ°Ζ¥”ΠΙΐ≥Χ÷–Θ§ΡΤ‘ΣΥΊ–Έ≥…ΝΥΈ»Ε®ΒΡΜ·ΚœΈοΘ§»ή“Κ÷–ΜΙ…ζ≥…ΝΥ“Μ÷÷≤ΜΚήΈ»Ε®ΓΔΨΏ”–Τ·ΑΉ–‘ΒΡΈο÷ XΘ§XΒΡΜ·―ß Ϋ « ΓΘ
Θ®3Θ©Ω…”Ο”“ΆΦΉΑ÷ΟΕ‘»ή“Κ÷–≤ΜΚήΈ»Ε®ΒΡΈο÷ Ϋχ––ΧΫΨΩΘ§‘ΎΔΌ¥ΠΉΑ»κΒΡΈο÷ « Θ®Χν―ΓœνΘ§œ¬Ά§Θ©Θ§ΔΎ¥ΠΉΑ»κΒΡΈο÷ « ΓΘ
AΘ°Ζ”ΧΣ ‘ΦΝ BΘ°Ιΐ―θΜ·ΡΤ”κΥ°Ζ¥”ΠΚσΒΡ»ή“Κ
CΘ°Εΰ―θΜ·ΟΧ DΘ°«β―θΜ·ΡΤΙΧΧε≈δ÷ΤΒΡ»ή“Κ
Θ®4Θ©ΤχΧεΔέ « Θ§Ιΐ―θΜ·ΡΤ”κΥ°Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΟΜ”––¥≥ωXά¥Θ§‘≠“ρ « ΓΘ


 CuCl42-(ΜΤ…Ϊ)+4H2OΓΘ
CuCl42-(ΜΤ…Ϊ)+4H2OΓΘ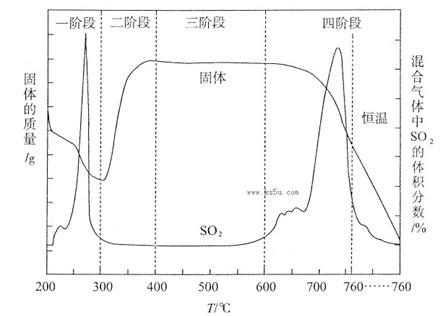

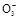
 BaCO3Γΐ+H2O)
BaCO3Γΐ+H2O)